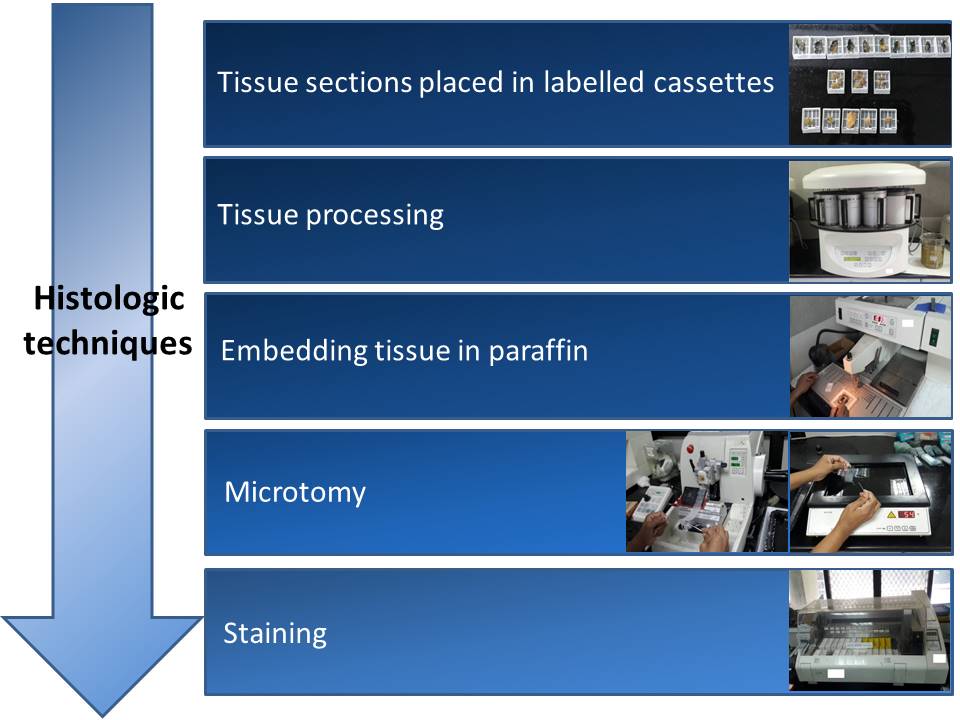
Tissue is prepared for microscopic examination by submitting sections of the tissue taken during grossing to a series of processes: fixation, dehydration, clearing, embedding, cutting, and staining.
Tissue processing
All the cassettes in which sections are submitted after grossing are placed in a tissue processor. In the carousel model of automatic tissue processor, tissues submitted in the cassettes are placed in the carrier basket and rotated through a series of stationary reagents arranged in a circular carousel. Processing schedules are flexible and should be standardized by the laboratory depending on the size and type of tissue processing. An example of one such schedule is:
- 10% Neutral-buffered formalin (5 minutes)
- 50% Ethyl alcohol (1 hour)
- 70% Ethyl alcohol (1 hour)
- 95% Ethyl alcohol (1 hour)
- 100% Ethyl alcohol (2 hours)
- 100% Ethyl alcohol (2 hours)
- 100% Ethyl alcohol (2 hours)
- Xylene (1 hour)
- Xylene (1 hour)
- Xylene (1 hour)
- Paraffin wax (2 hours)
- Paraffin wax (2 hours).
Reagents used for tissue processing
The reagents used for tissue processing include the following:
- Formalin fixes or stops decomposition of tissue.
- Different concentrations of alcohol (70%, 80%, 95%, and 100% in that sequence) dehydrate the tissue slowly.
- Xylene is the clearing reagent that removes the alcohol from the tissue to make the tissue ready for impregnation with paraffin.
- Liquid paraffin replaces the xylene and permanently infiltrates the tissue.
Manual (hand) tissue processing
Manual tissue processing is used only in the case of power failure or if an automatic tissue processor has broken. Manual processing is a tedious procedure that requires a dedicated technician. The tissue cassettes are placed in each reagent for the specified duration and sequentially according to the following schedule:
- 70% 2-Propanol (isopropanol) (30 minutes)
- 100% 2-Propanol (isopropanol) (1 hour)
- 100% 2-Propanol (isopropanol) (1 hour)
- 100% 2-Propanol (isopropanol) (1 hour)
- 100% 2-Propanol (isopropanol) (1 hour)
- 100% 2-Propanol (isopropanol) (1 hour)
- 100% 2-Propanol (isopropanol) (1 hour)
- Xylene (1 hour)
- Xylene (1 hour 30 minutes)
- Paraffin wax (2 hours)
- Paraffin wax (2 hours).
Points to remember when doing manual tissue processing
- It takes 13 hours to complete the schedule so it may not be possible to complete it within routine working hours. Because of this, it is important to plan the procedure as early as possible in the day. If necessary, step 10 (impregnation with paraffin wax) is shortened to 1 hour and the tissue cassettes are removed from the paraffin wax. Thereafter on the next day, steps 10 and 11 are continued to complete the entire process.
- An incubator set at 37°C is advisable for clearing in xylene.
- Paraffin impregnation should be carried out at 58°C in an oven.
|
Embedding tissue in paraffin
The plastic cassette that contains the pieces of processed tissue is taken to an embedding station. The tissue is removed from the cassette and placed in a mould filled with liquid paraffin.
The part of the cassette that has the patient’s identification number written or printed on it is then placed on top of the mould. After cooling, the cassette top and the paraffin-embedded tissue become a single unit, known as a paraffin block.
Microtomy
Microtomy is the procedure of cutting thin section of tissue to be placed on glass slides.
The paraffin block is placed in the block holder of the microtome, trimmed, and then several sections 5 µm thick are cut in long ribbons. These ribbons are floated on a bath of warm water. A glass slide is slipped under the section and it is lifted from the water onto the slide.
Staining the section with H&E
After drying in an oven (at 60°C), the slide is passed through another series of chemical reagents (listed below). Xylene removes the paraffin and absolute alcohol removes the xylene. The tissue on the slide is then rehydrated to prepare it for H&E staining.
The slides can then be examined under the microscope by a pathologist.
H&E staining for histopathology
Procedure:
- Place the slide with the section in an oven (5 minutes).
- Place in xylene (5 minutes).
- Place in xylene (5 minutes).
- Hydrate the section by placing in absolute alcohol (2 minutes).
- Hydrate the section by placing in 80% alcohol (30 seconds).
- Rinse in running tap water (1˝ minutes).
- Stain with haematoxylin (15 minutes).
- Rinse in running tap water (1˝ minutes).
- Dip in 1% acid alcohol (2 seconds).
- Rinse in running tap water (10 minutes).
- Stain with eosin (4 minutes).
- Rinse in running tap water (1˝ minutes).
- Dehydrate the section by placing in absolute alcohol (1 minute).
- Dehydrate the section by placing in absolute alcohol (1 minute).
- Clear in xylene.
- Mount in DPX.
Practice points in processing core needle biopsy
- The cores should be placed in 10% neutral-buffered formalin (pH 7.4) immediately after removal and sent promptly to the laboratory.
- Optimal time in formalin fixation for accurate biomarker testing is 6–72 hours.
- While grossing, record the number of cores and length of each core. When handling the biopsies avoid any crushing with forceps.
- Biopsies for calcification need to be radiographed by the radiologist to demonstrate that the targeted calcification has been removed.
- The cores should be embedded as closely as possible in the same plane such that all levels on cutting will be representative of the cores. The cores should not overlap.
- When cutting the paraffin block use three spaced levels such that the deepest level is about half way through the block. This provides a good sampling of the tissue and can be used for H&E staining.
- The levels in between the above mentioned three levels can be taken for IHC, for biomarker testing.
- For mass-forming lesions, diagnostic tissue is present in the first level in 98% of cases. Lesions removed for calcifications on mammography are generally smaller and usually require more levels of cutting.
|
|   |
|
.png)