Home / Reseach Projects / Cancer control
Legend: Ongoing projects (blue), Completed projects (green)
Cancer control
| Study sites: | Ireland, Lithuania, Poland, Spain (Manresa, Galicia) |
| Principal investigator (PI) from IARC: | A. Chandran, P. Basu |
| PIs from collaborating institutions: |
|
| Start date: | 2023 |
| Closure date: | Ongoing |
| Objectives: | The PRAISE-U project aims to reduce morbidity and mortality caused by prostate cancer in EU Member States through smart early detection. In partnership with the consortium, PRAISE-U works to encourage early detection and diagnosis of prostate cancer through customised and risk-based screening programmes. The goal is to align protocols and guidelines across Member States and enable the collection and distribution of relevant data to reduce prostate cancer morbidity and mortality rates in Europe. |
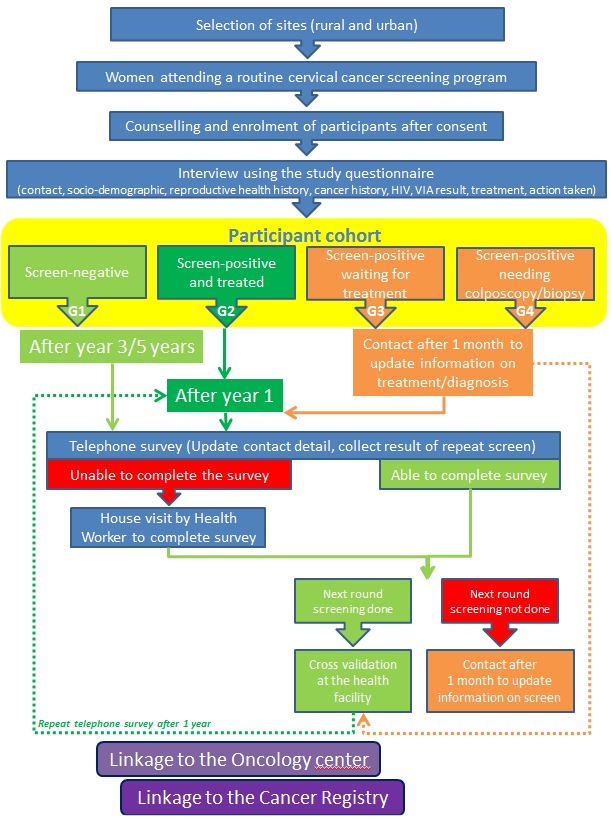
| Methodology: | 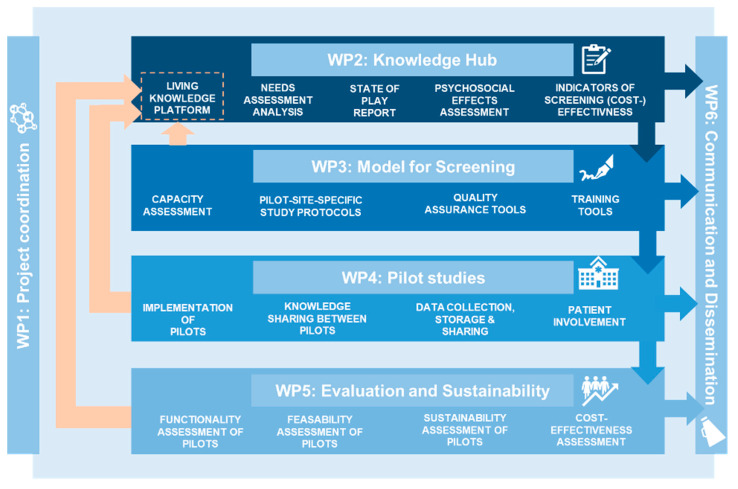 Work Package 1 acts as the template for the overview, coordination and quality assurance of the project. Progress will be assessed by tracking the timely completion of milestones and deliverables, and the monitoring of planned resources. Work Package 2 acts as the foundation to Work Packages 3, 4, and 5 by developing a living state of play document, a needs assessment report, performance indicators, and the Platform Knowledge Hub on Prostate Cancer screening. Work Package 3 will build upon the results of Work Package 2 to design the protocols and other research tools for the different pilots to evaluate the risk based screenings. Work Package 4 is the setup and coordination of pilot studies in the host countries. The objectives of this is to facilitate the sharing of knowledge between the pilot sites and core scientific team, ensure the implementation of the agreed protocol and tools, and streamline data collection and monitoring of indicators. Work Package 5 will assess the functionality, feasibility, and sustainability of screening pilots by analysing performance of all the pilot sites, comparisons between pilot sites, and comparisons with the pre-pilot situation, using amongst others, the performance indicators as defined in Work Package 2. Work Package 6 runs in parallel with the other Work packages during the entire lifecycle of the project, focusing on the communication and dissemination of the project's aims, approach and results. Work Package 6 will support all partners to ensure information flow from other Work Packages and to enable for external dissemination and communication. Its key purpose is to ensure that all relevant information. | Publications: | Van Poppel H., Roobol M.J., Chandran A. Early Detection of Prostate Cancer in the European Union: Combining Forces with PRAISE-U. Eur Urol. 2023 Dec;84(6):519-522. doi: 10.1016/j.eururo.2023.08.002. Epub 2023 Sep 12. PMID: 37704541 Gómez Rivas J., Leenen R.C.A., Venderbos L.D.F., Helleman J., de la Parra I., Vasilyeva V., Moreno-Sierra J., Basu P., Chandran A., van den Bergh R.C.N., Collen S., Van Poppel H., Roobol M.J., Beyer K., On Behalf Of The Praise-U Consortium. Navigating through the Controversies and Emerging Paradigms in Early Detection of Prostate Cancer: Bridging the Gap from Classic RCTs to Modern Population-Based Pilot Programs. J Pers Med. 2023 Dec 1;13(12):1677. PMID: 38138904 |
| Funding: | The project leading to this application has received funding from the EU4Health programme under Grant Agreement n° 101101217 |
| Study sites: | Not applicable |
| Principal investigator (PI) from IARC: | P. Basu and A. Carvalho |
| PIs from collaborating institutions: |
|
| Start date: | 2017 |
| Closure date: | Ongoing |
| Objectives: | The purpose of the project is to collect standardized information on the characteristics and performance of cancer screening programmes around the world, and to disseminate that information to enable improved programme management and informed policy, strengthen health information systems, and support research. Systematic, standardized reporting of programme characteristics and quality indicators will be of great value not only to the participating programme managers to improve programme quality, but also to others striving to make their programmes more effective. CanScreen5 will be an online portal for the collection, analysis, and dissemination of information on cancer screening programmes and activities in countries around the world, with the core objective of motivating and enabling countries to collect and use cancer screening data in a consistent manner. |
| Methodology: | 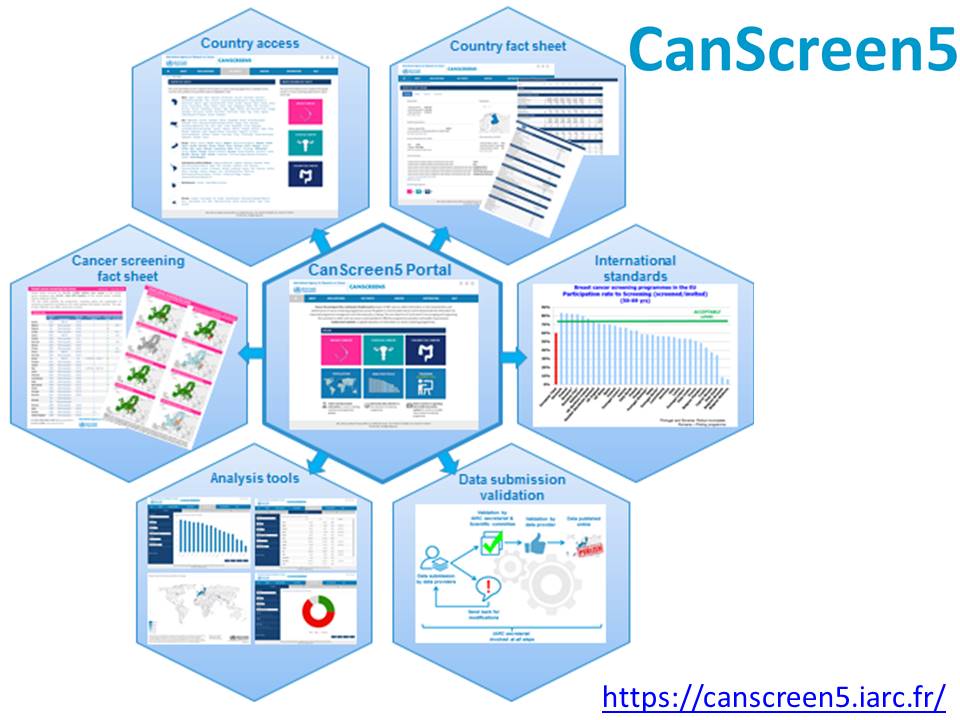 The project methodology will be based on the IARC Screening Group’s recent experience reporting the status of implementation and performance of breast, cervical, and colorectal cancer screening programmes in the 28 member states of the European Union (“Cancer Screening in the European Union”, 2017). A network of collaborators and institutions will be formed in order to access qualitative and quantitative information on cancer screening programmes in various countries. A protocol for standardized data collection will be developed, along with data collection tools. A web-based platform will be created for data collection and dissemination of the analysed data. The IARC Screening Group will serve as the project secretariat, and the project will be implemented in phases. Quality checks will be carried out to ensure that the data collected from each country meet a defined set of minimum standards. Ethical issues, protection of data privacy, and regulatory issues that apply to sharing data will be thoroughly reviewed. Visit the CanScreen5 website. |
| Funding: | Intramural funding from IARC; support from the Pan American Health Organization (PAHO) |
| Study sites: | East Africa |
| Principal investigator (PI) from IARC: | P. Basu and A. Carvalho |
| Start date: | 2020 |
| Closure date: | Ongoing |
| Objectives: | We aim to implement CanScreen5 project in selected East African countries through a partnership with Cancer Research Council UK.
Specific objectives are:
|
| Methodology: | Training of the screening programme managers Under the framework of CanScreen5 project, the first training programme for selected African countries is being organized at IARC, as this will allow identification of quality indicators most relevant for the region, developing regional standards and ultimately regional implementation guidelines for the project. For the first training we invited representatives from the following countries: Angola, Botswana, Cameroon, Ethiopia, Eritrea, Ghana, Kenya, Malawi, Mozambique, Namibia, Nigeria, Rwanda, South Africa, Uganda, United Republic of Tanzania, Zambia, and Zimbabwe. The participants have been nominated by the Ministry of Health of each country. We anticipate that at the completion of the course, participants will be able to:
CanScreen5 data will allow us to understand the protocol, organization and quality of the cancer screening programmes and also estimate some of the key performance indicators of screening. Based on the data collected regarding cancer screening through CanScreen5 project, we will select 3 African countries to perform an in-depth situation analysis with the following specific objectives:
|
| Funding: | Medical Research Council UK |
| Study sites: | Community of Caribbean and Latin American States (CELAC): Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Bolivia (Plurinational State of), Brazil, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, El Salvador, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay, Peru, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Uruguay and Venezuela (Bolivarian Republic of) |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Start date: | 2019 |
| Closure date: | Ongoing |
| Objectives: |
|
| Methodology: | The study will be conducted in two simultaneous phases:
|
| Funding: | Norwegian Research Council |
| Study sites: | Afghanistan, Bangladesh, Burundi, Cameroon, Central African Republic, Chad, Congo Democratic Republic, Congo Republic, Cote d'Ivoire, Democratic People’s Republic of Korea, Eritrea, Ethiopia, Guinea, Guinea Bissau, Haiti, Iraq, Kenya, Lebanon, Liberia, Libya, Malawi, Mali, Mauritania, Myanmar, Nepal, Niger, Nigeria, Pakistan, Sierra Leone, Somalia, South Sudan, Sri Lanka, Sudan, Syrian Arab Republic, Timor-Leste, Uganda, Uzbekistan, Yemen and Zimbabwe |
| Principal investigator (PI) from IARC: | A. Carvalho |
| PIs from collaborating institutions: | André Ilbawi, World Health Organization |
| Start date: | 2020 |
| Closure date: | Ongoing |
| Objectives: |
|
| Methodology: | The selection of countries to be analysed is based on their score in the Fragility State Index (FSI), since its creation in 2006. Three groups of states will be assessed:
Additionally, trends in cancer incidence, mortality and disability adjusted life years (DALY) and overall health indicators (maternal mortality, HIV prevalence, etc.) will be compiled. An evaluation of the correlation of trends of the FSI and trends in cancer burden (measured by trends in incidence, mortality and DALYs) and overall health indicators will be conducted. |
| Funding: | Intramural funds from IARC |
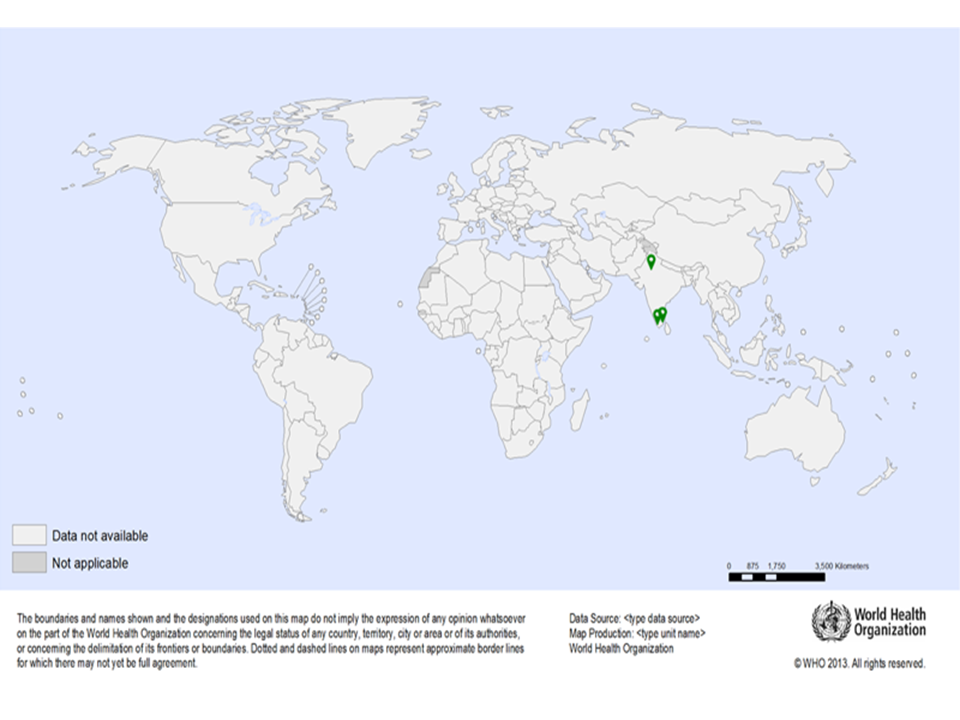
| Study sites: | Udaipur, India |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: | Manoj Mahajan (PI), Kirti Jain, Nilesh Patira, GBH Memorial Cancer Hospital, Udaipur, India |
| Map: |  |
| Start date: | 2017 |
| Closure date: | Ongoing |
| Objectives: | To evaluate the feasibility and acceptability of the model of delivering community health worker (CHW)–driven home-based comprehensive noncommunicable disease (NCD) control services aimed to prevent premature deaths from cardiovascular diseases; stroke; and breast, cervical, and oral cancers in hard-to-reach men and women |
| Methodology: | The implementation research has two main components, which will be conducted simultaneously:
| Publications: | Basu P., Mahajan M., Patira N., Prasad S., Mogri S., Muwonge R., Lucas E., Sankaranarayanan R., Iyer S., Naik N., Jain K. A pilot study to evaluate home-based screening for the common non-communicable diseases by a dedicated cadre of community health workers in a rural setting in India. BMC Public Health. 2019 Jan 3;19(1):14. PMID: 30606132 Mahajan M., Naik N., Jain K., Patira N., Prasad S., Mogri S., Muwonge R., Lucas E., Faruq F., Sankaranarayanan R., Iyer S., Basu P. Study of Knowledge, Attitudes, and Practices Toward Risk Factors and Early Detection of Noncommunicable Diseases Among Rural Women in India. J Glob Oncol. 2019 Apr;(5):1-10. PMID: 30998427 |
| Funding: | American International Health Management Ltd |
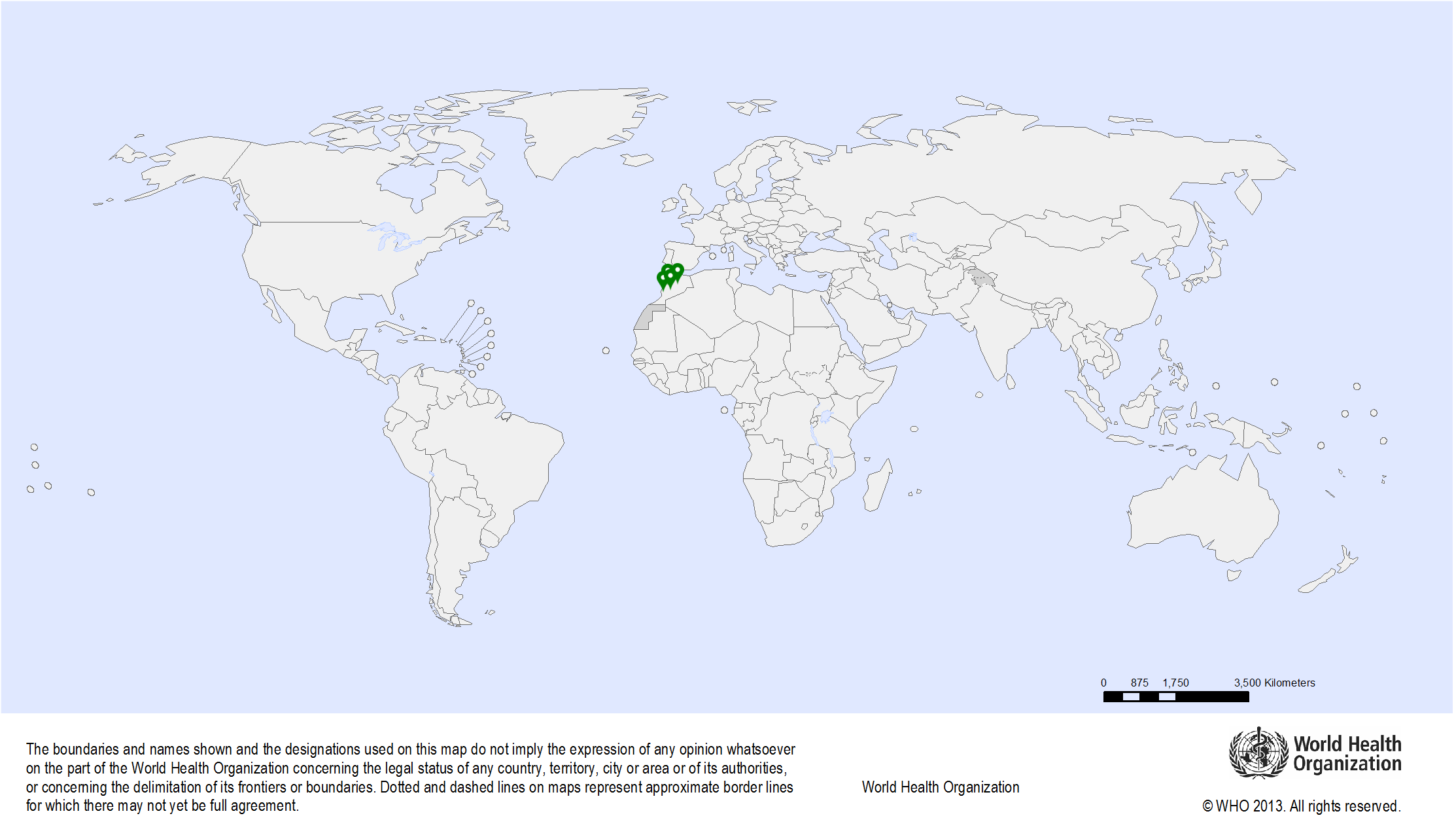
| Study sites: | Morocco: Casablanca, Fez, Rabat |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: | 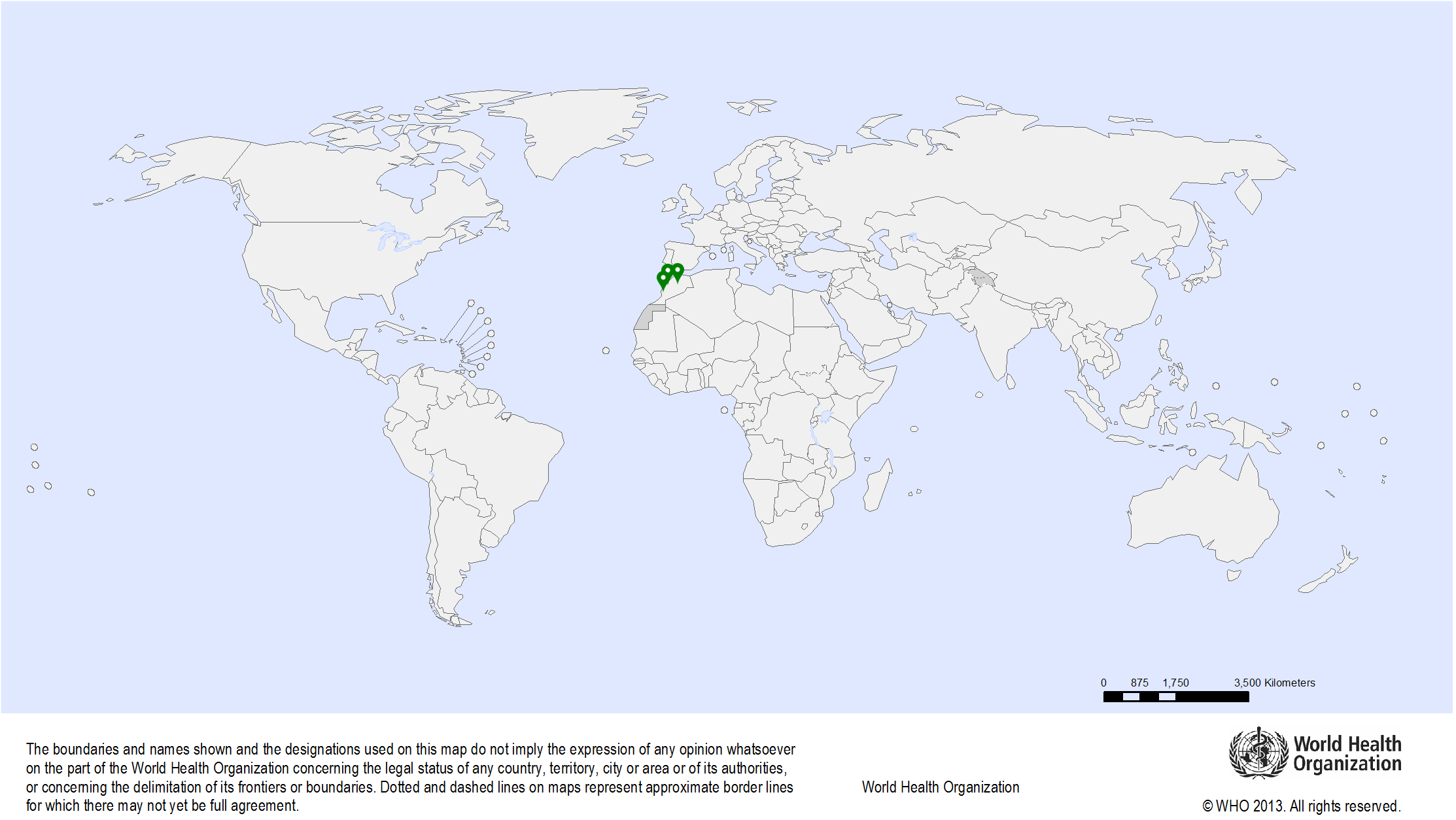 |
| Start date: | 2016 |
| Closure date: | 2019 |
| Objectives: |
|
| Methodology: | The evaluation will be based on information obtained through in-depth interviews of the key functionaries of the programme, focus group discussions with various categories of service providers, and supervisory visits to randomly selected facilities offering screening and diagnostic services. | Publications: | Basu P., Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C. Breast Cancer Screening Program in Morocco: Status of implementation, organization and performance. Int J Cancer. 2018 Jul 14. PMID: 30006933 Selmouni F., Zidouh A., Belakhel L., Sauvaget C., Bennani M., Khazraji Y.C., Benider A., Wild C.P., Bekkali R., Fadhil I., Sankaranarayanan R. Tackling cancer burden in low-income and middle-income countries: Morocco as an exemplar. Lancet Oncol. 2018 Feb;19(2):e93-e101. PMID: 29413484 Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C., Basu P. Evaluation of the national cervical cancer screening program in Morocco: achievements and challenges. J Med Screen. 2019 Jan 16:969141318824627. PMID: 30651034 |
| Funding: | Lalla Salma Foundation for Cancer Prevention and Treatment |
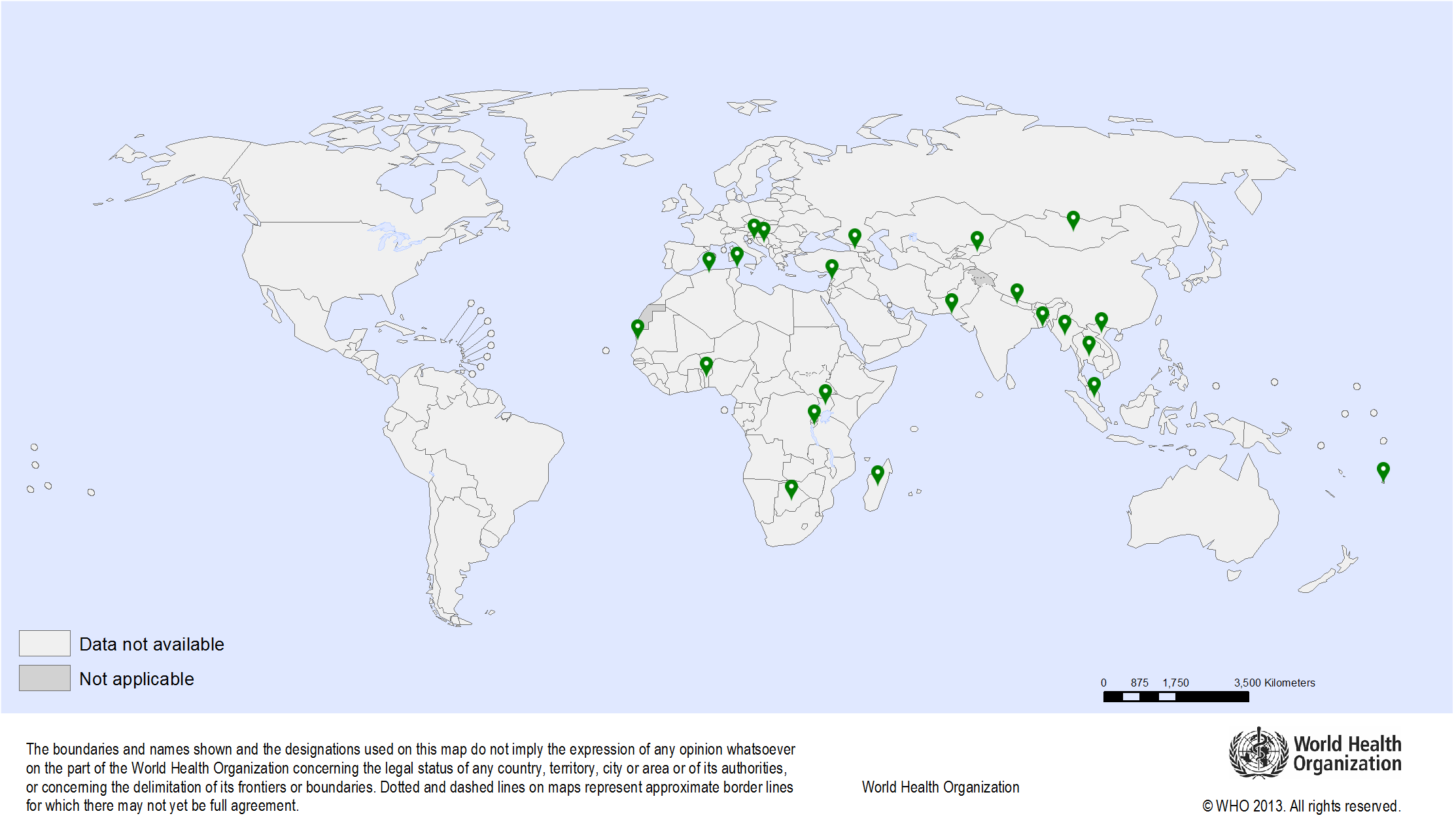
| Study sites: |
|
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: | 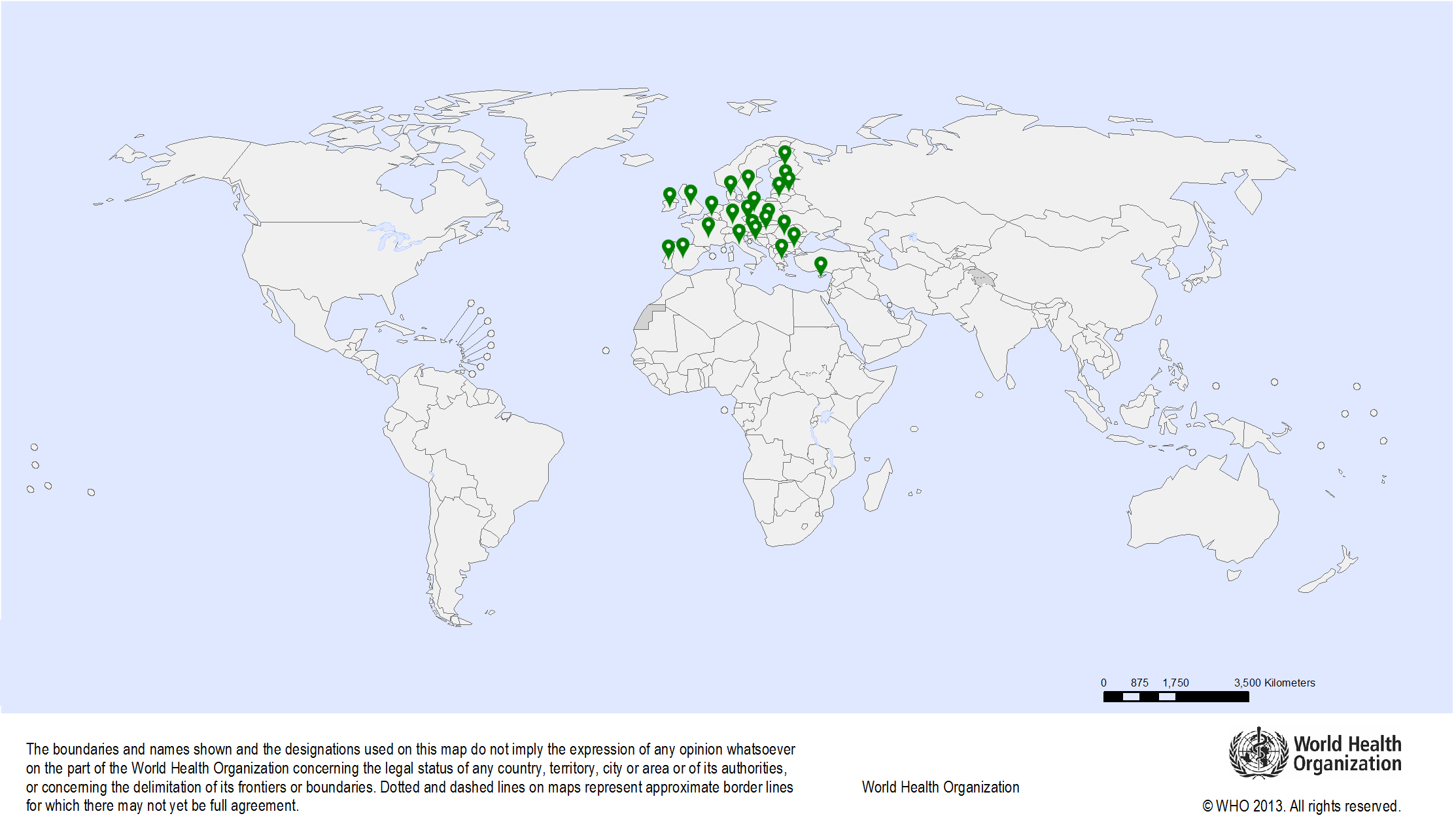 |
| Start date: | 2015 |
| Closure date: | 2017 |
| Objectives: |
|
| Methodology: |
| Publications: | Basu P., Ponti A., Anttila A., Ronco G., Senore C., Vale D. B., Segnan N., Tomatis M., Soerjomataram I., Primic Zakelj M., Dillner J., Elfstrom K. M., Lonnberg S., Sankaranarayanan R. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer; 2018; 142(1): 44-56. PMID: 28940326 Basu P., Ponti A., Anttila A., Ronco G., Senore C., Vale D.B., Segnan N., Tomatis M., Soerjomataram I., Primic Žakelj M., Dillner J., Elfström K.M., Lönnberg S., Sankaranarayanan R. Author's reply to: Cancer screening policy in Hungary. Int J Cancer. 2018 Aug 15;143(4):1005. PMID: 29524204 Basu P., Ponti A., Anttila A., Ronco G., Senore C., Vale D.B., Segnan N., Tomatis M., Soerjomataram I., Žakelj M.P., Dillner J., Elfström K.M., Lönnberg S., Sankaranarayanan R. Author's reply to: Implementation and organization of cancer screening in France. Int J Cancer. 2018 Jun 26. doi: 10.1002/ijc.31629. PMID: 29943811 Senore C., Basu P., Anttila A., Ponti A., Tomatis M., Vale D.B., Ronco G., Soerjomataram I., Primic-Žakelj M., Riggi E., Dillner J., Elfström M.K., Lönnberg S., Sankaranarayanan R., Segnan N. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2018 Dec 10. PMID: 30530530 Vale D.B., Anttila A., Ponti A., Senore C., Sankaranaryanan R., Ronco G., Segnan N., Tomatis M., Žakelj M.P., Elfström K.M., Lönnberg S., Dillner J., Basu P. Invitation strategies and coverage in the population-based cancer screening programmes in the European Union. Eur J Cancer Prev. 2018 Mar 21. PMID: 29570103 View the report |
| Funding: | The European Union Public Health Programme (scientific and technical support to the European Partnership for Action Against Cancer) |
| Study sites: | Dhaka, Bangladesh |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Dr Ashrafun Nessa, Bangabandhu Sheikh Mujib Medical University (BSMMU) |
| Map: | 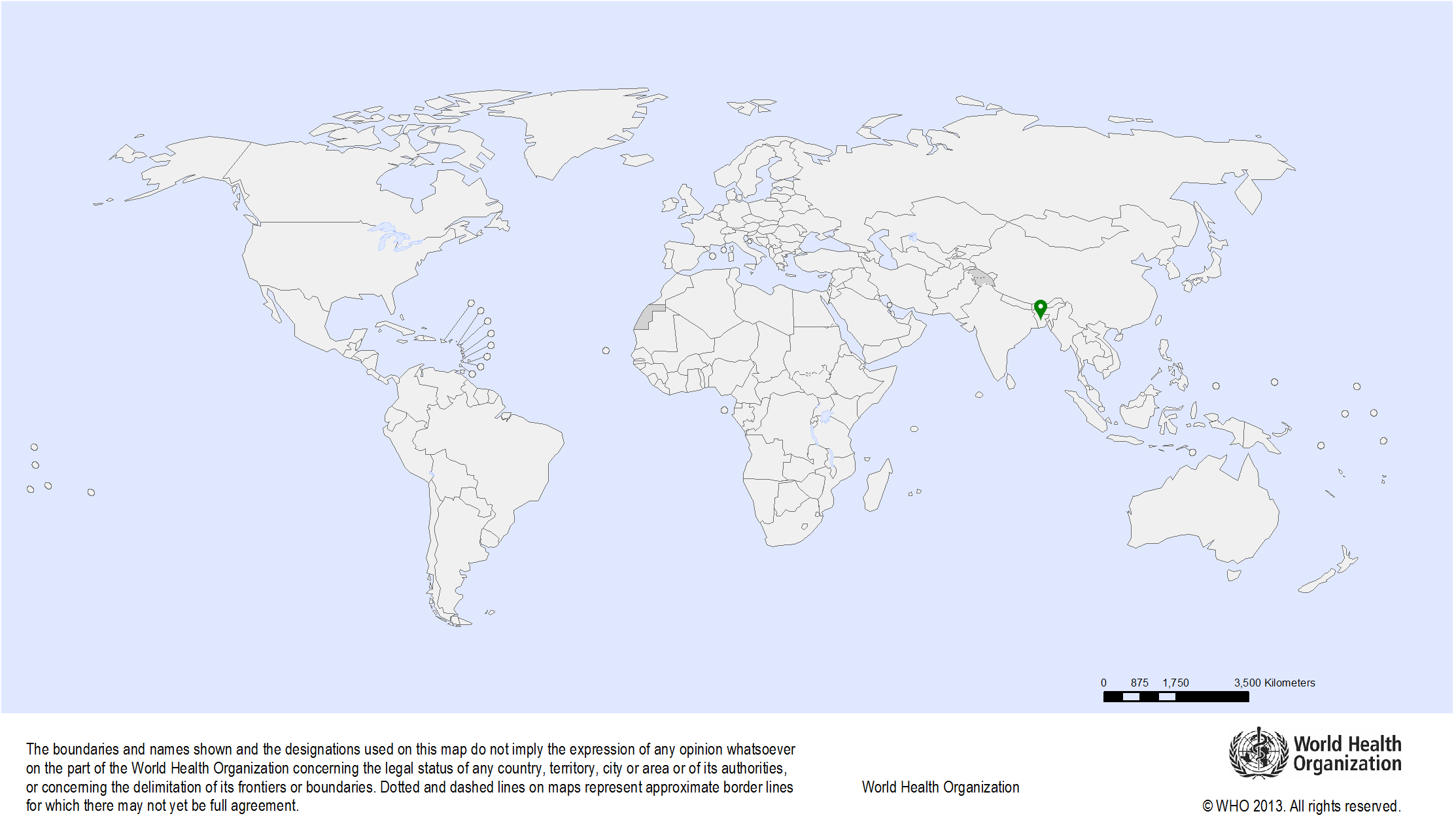 |
| Start date: | 2006 |
| Closure date: | 2017 |
| Objectives: | To provide technical support to help organize a screening programme for cervical cancer prevention in Bangladesh |
| Methodology: | The following technical support is provided:
| Publications: | Nessa A., Hussain M.A., Rahman J.N., Rashid M.H., Muwonge R., Sankaranarayanan R. Screening for cervical neoplasia in Bangladesh using visual inspection with acetic acid. Int J Gynaecol Obstet. 2010;111(2):115-8. PMID: 20674919 Sankaranarayanan R., Bhatla N., Gravitt P.E., Basu P., Esmy P.O., Ashrafunnessa K.S., Ariyaratne Y., Shah A., Nene B.M. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26(Suppl 12):M43-M52. PMID: 18945413 |
| Study sites: | National Cancer Institute / National Cancer Control Programme, Sri Lanka |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Suraj Perera |
| Map: | 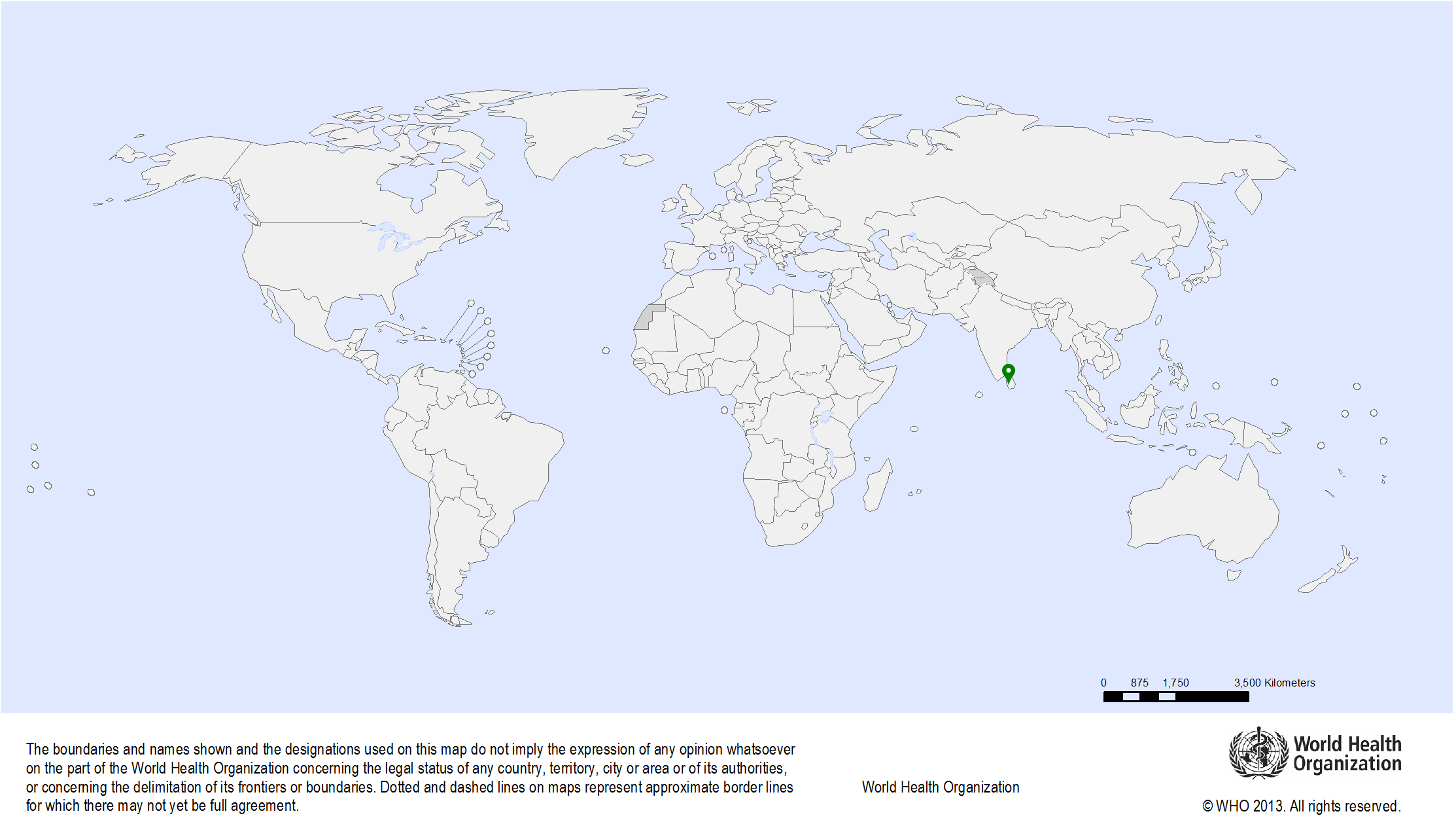 |
| Start date: | 2006 |
| Closure date: | 2017 |
| Objectives: | To provide technical support to the national cancer control programme |
| Methodology: | The following technical support is provided:
| Publications: | Sankaranarayanan R., Bhatla N., Gravitt P.E., Basu P., Esmy P.O., Ashrafunnessa K.S., Ariyaratne Y., Shah A., Nene B.M. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26(Suppl 12):M43-M52. PMID: 18945413 |
| Study sites: | Thailand |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Weerawut Imsamran, Suleeporn Sangrajrang, Pattarawin Attasara, National Cancer Institute, Bangkok, Thailand |
| Map: |  |
| Start date: | 2006 |
| Closure date: | 2017 |
| Objectives: | To provide technical support to the national cancer control programme |
| Methodology: | The following technical support is provided:
| Publications: | Deerasamee S., Srivatanakul P., Sriplung H., Nilvachararung S., Tansuwan U., Pitakpraiwan P., Kaewkungwal J., Singhasivanon P., Nimnakorn P., Sankaranarayanan R. Monitoring and evaluation of a model demonstration project for the control of cervical cancer in Nakhon Phanom province, Thailand. Asian Pac J Cancer Prev. 2007 Oct-Dec;8(4):547-56. PMID: 18260727 Khuhaprema T., Attasara P., Srivatanakul P., Sangrajrang S., Muwonge R., Sauvaget C., Sankaranarayanan R. Organization and evolution of organized cervical cytology screening in Thailand. Int J Gynaecol Obstet. 2012;118(2):107-11. PMID: 22613493 |
| Study sites: | Ministry of Health, Morocco |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2014 |
| Closure date: | 2017 |
| Objectives: | To provide technical support to improve the current information system for monitoring the breast and cervical cancer screening programme |
| Methodology: | We propose for the Breast and Cervical Cancer Early Detection Programme
| Publications: | Basu P., Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C. Breast Cancer Screening Program in Morocco: Status of implementation, organization and performance. Int J Cancer. 2018 Jul 14. PMID: 30006933 Selmouni F., Zidouh A., Belakhel L., Sauvaget C., Bennani M., Khazraji Y.C., Benider A., Wild C.P., Bekkali R., Fadhil I., Sankaranarayanan R. Tackling cancer burden in low-income and middle-income countries: Morocco as an exemplar. Lancet Oncol. 2018 Feb;19(2):e93-e101. PMID: 29413484 |
| Funding: | United Nations Population Fund (UNFPA) Morocco |
| Study sites: |
|
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | R. Swaminathan, H. Brenner, B. Ganesh, A. Mathew, M. Hakama, K. Jayant |
| Map: |  |
| Start date: | 1990 |
| Closure date: | 2011 |
| Objectives: | The dearth of reliable survival statistics from developing countries was very evident until the mid-1990s. This prompted IARC to undertake a project that facilitated hands-on training and thereby the transfer of knowledge and technology on cancer survival analysis to researchers from the participating population-based cancer registries, which culminated in the publication of the first volume of the IARC Scientific Publication on cancer survival in developing countries in 1998. The present study is the second in the series, with wider geographical coverage, and is based on data from 27 registries in 14 countries in Africa, Asia, the Caribbean, and Central America. The calendar period of registration of incident cases for the present study ranges between 1990 and 2001. Data on 564 606 cases of 1–56 cancer sites from different registries are reported. Data from 11 registries were used to determine survival trends and data from 17 registries for reporting survival by clinical extent of disease. The publication includes chapters on each registry and general chapters on methodology, the database, and an overview. Comparative statistics on cancer survival by participating registry or by cancer site are also available online in the form of tables and graphs (https://survcan.iarc.fr). |
| Methodology: | Population-based cancer survival data (a key indicator for monitoring progress against cancer) are not widely available in low- and middle-income countries. Cancer-specific survival of patients diagnosed in 1990–2001, and followed-up to 2003 in 25 population-based cancer registries from 12 countries in sub-Saharan Africa, Central America, and Asia were analysed by actuarial methods. Visit the SurvCan website | Publications: | Sankaranarayanan R., Swaminathan R. Cancer survival in Africa, Asia, the Caribbean and Central America. Lyon: International Agency for Research on Cancer. IARC Scientific Publication No. 162; 2011. Visit the book’s IARC Publications page Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G., Law S.C., Ahn Y.O., Xiang Y.B., Yeole B.B., Shin H.R., Shanta V., Woo Z.H., Martin N., Sumitsawan Y., Sriplung H., Barboza A.O., Eser S., Nene B.M., Suwanrungruang K., Jayalekshmi P., Dikshit R., Wabinga H., Esteban D.B., Laudico A., Bhurgri Y., Bah E., Al-Hamdan N. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165-73. PMID: 20005175 |
| Funding: |
|
Legend: Ongoing projects (blue), Completed projects (green)
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85
© IARC 2026 - Terms of use - Privacy Policy.
© IARC 2026 - Terms of use - Privacy Policy.