Home / Reseach Projects / Breast cancer early detection
Legend: Ongoing projects (blue), Completed projects (green)
Research projects
Breast cancer early detection
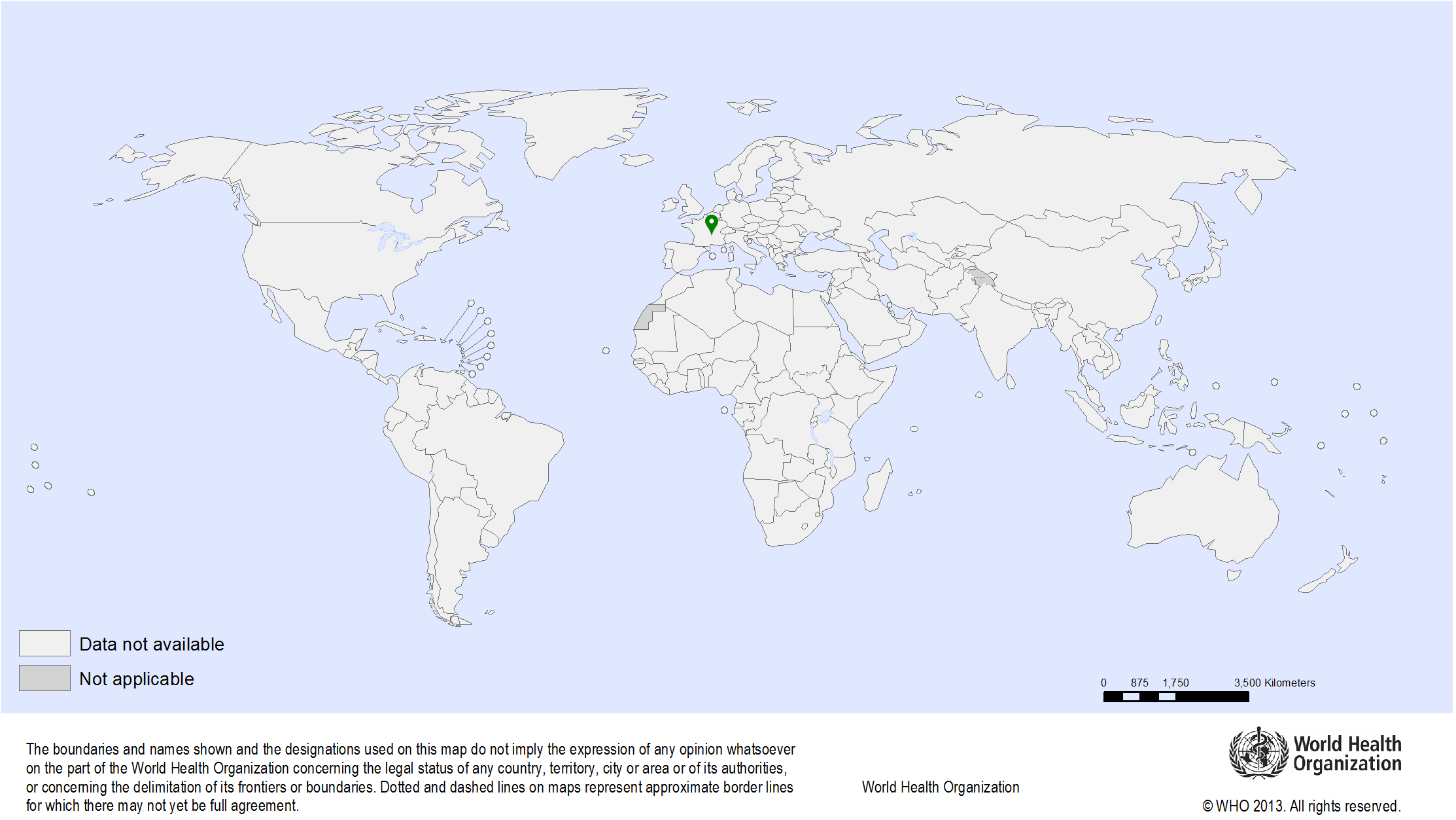
| Study sites: | Thiruvananthapuram (formerly called Trivandrum), Kerala, India |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: | K. Ramadas (PI), S. Thara, Beela Sarah Mathew, Elizabeth Abraham, Gigi Thomas, Jem Prabhakar, Paul Augustine, Ramani Wesley, R. Rejnish Kumar; Regional Cancer Centre (RCC), Thiruvananthapuram, Kerala, India |
| Map: |  |
| Start date: | 2006 |
| Closure date: | Ongoing |
| Objectives: | To evaluate whether three rounds of triennial clinical breast examination (CBE) can reduce the incidence rate of advanced breast cancers and reduce the rate of mortality from breast cancer |
| Methodology: | |
| Study outcomes: | This community-based cluster randomized controlled trial involving 120 000 women evaluates the role of intervention packages consisting of breast awareness, health education, opportunities for early clinical diagnosis (i.e. CBE), and the provision of readily accessible diagnosis and treatment services for the early detection and improved outcome of breast cancer. The intervention trial is being conducted in 14 administrative regions (“panchayats”) in Thiruvananthapuram District, Kerala, India. Approximately 120 000 households and about 120 000 women aged 30–69 years in the two sub-districts were grouped into about 275 clusters based on wards; 133 clusters were randomized to the intervention group to receive CBE. The other 142 clusters were randomized to the control group to receive the existing usual health care and health education on early detection of breast cancer. We have already demonstrated significant downstaging of breast cancers through CBE screening. The 10-year follow-up of the cohorts revealed the high negative predictive value of CBE: the invasive breast cancer detection rate in the CBE-negative women was very low. | Publications: |  Sankaranarayanan R., Ramadas K., Thara S., Muwonge R., Prabhakar J., Augustine P., Venugopal M., Anju G., Mathew B.S. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103(19):1476-80. Sankaranarayanan R., Ramadas K., Thara S., Muwonge R., Prabhakar J., Augustine P., Venugopal M., Anju G., Mathew B.S. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103(19):1476-80.PMID: 21862730  Grosse Frie K., Ramadas K., Anju G.A., Mathew B., Muwonge R., Sauvaget C., Thara S., Sankaranarayanan R. Determinants of participation in a breast cancer screening trial in Trivandrum District, India. Asian Pac J Cancer Prev. 2013;14(12):7301-7. Grosse Frie K., Ramadas K., Anju G.A., Mathew B., Muwonge R., Sauvaget C., Thara S., Sankaranarayanan R. Determinants of participation in a breast cancer screening trial in Trivandrum District, India. Asian Pac J Cancer Prev. 2013;14(12):7301-7.PMID: 24460292 |
| Funding: | Intramural funds from IARC | Media: |
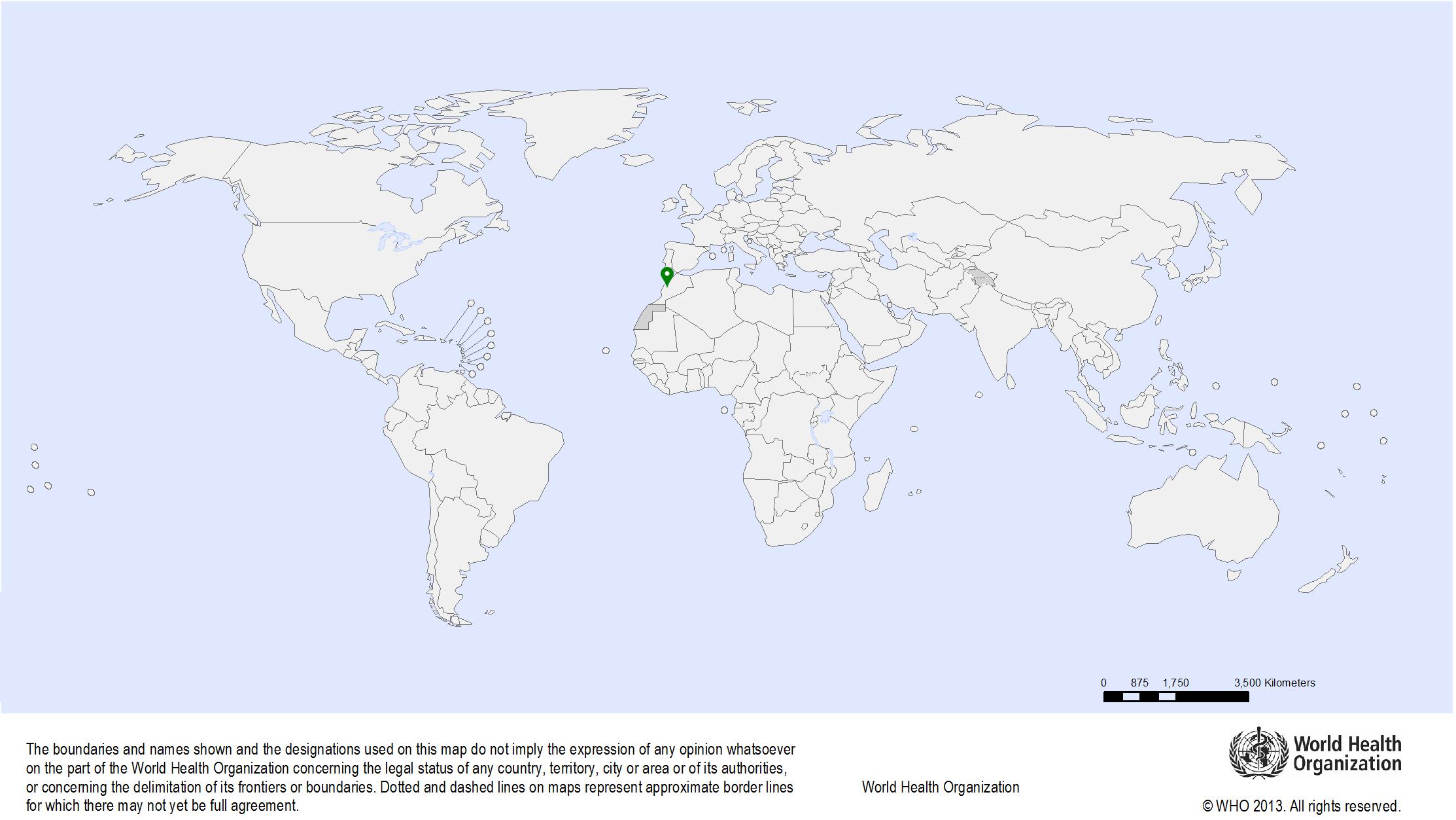
| Study sites: | Morocco: Casablanca, Fez, Rabat |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2016 |
| Closure date: | Ongoing |
| Objectives: | To evaluate the quality of care for women diagnosed with breast and cervical cancer within the public health delivery system of Morocco |
| Methodology: |  The basis of the study is a questionnaire survey based on medical charts. The key information that will be obtained from this retrospective study includes information regarding the mode of diagnosis, the gap between the onset of symptoms and diagnosis, the waiting time for start of treatment, and the factors influencing delays in diagnosis and initiation of treatment. The study will also gather information on the protocol followed for the diagnosis and treatment of breast and cervical cancers in the leading oncology centres in Morocco, and on the patients’ adherence to such protocols. Information on the quality of recordkeeping in the institutions will also be gathered from the study. The publication Patterns of care for women with breast cancer in Morocco: an assessment of breast cancer diagnosis, management, and survival in two leading oncology centres summarizes the outcomes of a patterns-of-care study implemented by IARC in collaboration with the Ministry of Health of the Kingdom of Morocco and the Lalla Salma Foundation for Cancer Prevention and Treatment, to assess how far state-of-the-art cancer diagnostics and therapy have been disseminated into routine oncology practice in Morocco. The findings highlight the improvements in breast cancer care that occurred in Morocco as a result of pragmatic policies and systematic planning by the Moroccan Ministry of Health. Recommendations for strengthening breast cancer care in Morocco are also included in the publication. View the publication, and the IARC news. | Publications: |  Thankappan K., Subramanian S., Balasubramanian D., Kuriakose M.A., Sankaranarayanan R., Iyer S. Cost-effectiveness of oral cancer screening approaches by visual examination: A systematic review. Head Neck. 2021 Jul 14. Thankappan K., Subramanian S., Balasubramanian D., Kuriakose M.A., Sankaranarayanan R., Iyer S. Cost-effectiveness of oral cancer screening approaches by visual examination: A systematic review. Head Neck. 2021 Jul 14.PMID: 34260118  Basu P., Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C. Breast Cancer Screening Program in Morocco: Status of implementation, organization and performance. Int J Cancer. 2018 Jul 14. Basu P., Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C. Breast Cancer Screening Program in Morocco: Status of implementation, organization and performance. Int J Cancer. 2018 Jul 14.PMID: 30006933  Selmouni F., Zidouh A., Belakhel L., Sauvaget C., Bennani M., Khazraji Y.C., Benider A., Wild C.P., Bekkali R., Fadhil I., Sankaranarayanan R. Tackling cancer burden in low-income and middle-income countries: Morocco as an exemplar. Lancet Oncol. 2018 Feb;19(2):e93-e101. Selmouni F., Zidouh A., Belakhel L., Sauvaget C., Bennani M., Khazraji Y.C., Benider A., Wild C.P., Bekkali R., Fadhil I., Sankaranarayanan R. Tackling cancer burden in low-income and middle-income countries: Morocco as an exemplar. Lancet Oncol. 2018 Feb;19(2):e93-e101.PMID: 29413484 |
| Funding: |
|
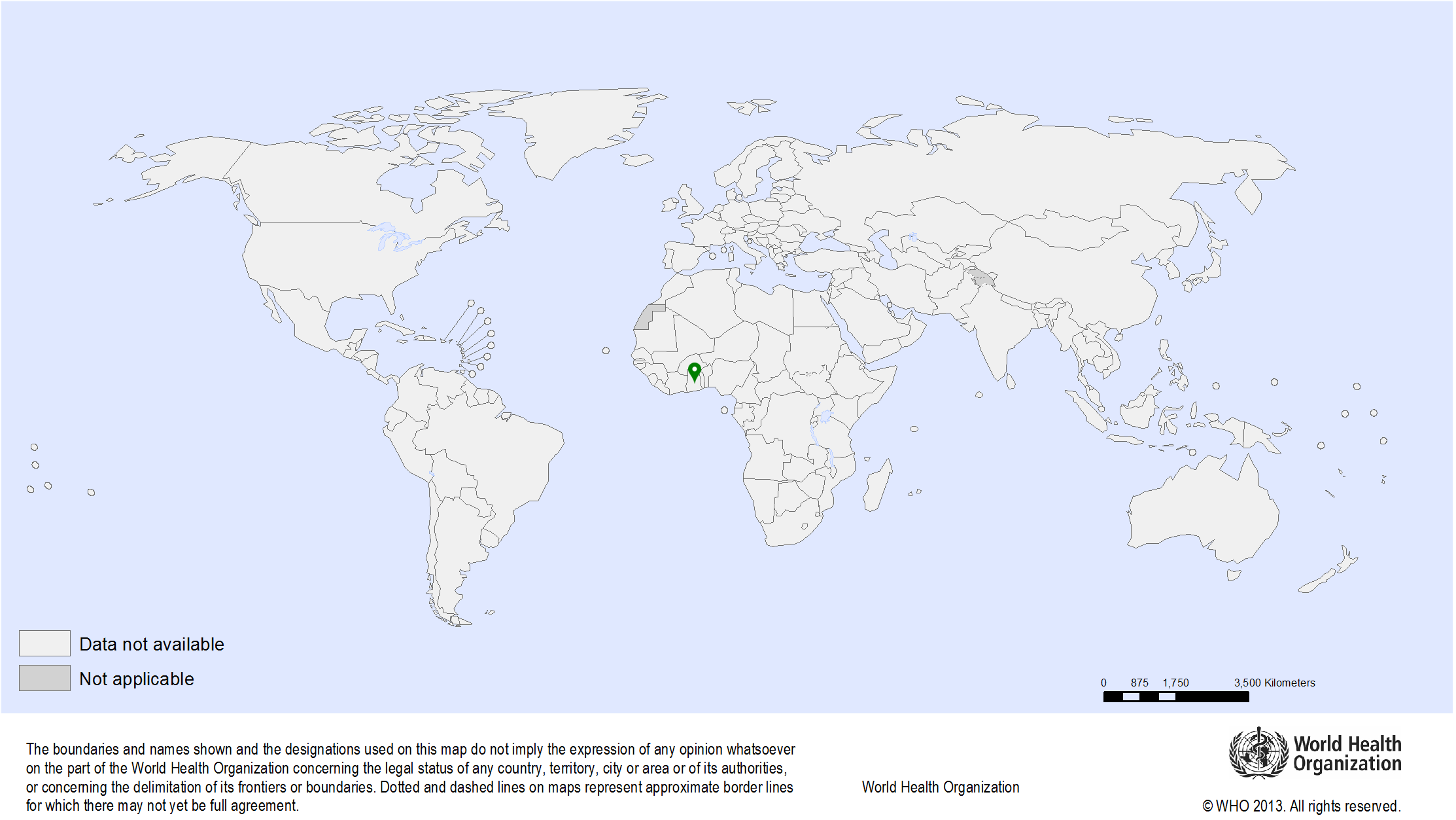
| Study sites: | Mumbai, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Anita Gadgil, Bhabha Atomic Research Centre (BARC) Hospital, Homi Bhabha National Institute University, Mumbai, India |
| Map: |  |
| Start date: | 2013 |
| Closure date: | 2019 |
| Objectives: | To implement a programme on breast cancer to increase awareness and early detection by clinical breast examination (CBE) among 22 500 women aged 30–69 years (members of a large health maintenance organization) in order to bring about breast cancer case downstaging, to offer different treatment modalities, and to achieve better survival and quality of life |
| Methodology: |
| Publications: |  Gadgil A., Roy N., Sankaranarayanan R., Muwonge R., Sauvaget C. Effect of comprehensive breast care on breast cancer outcomes: a community hospital based study from Mumbai, India. Asian Pac J Cancer Prev. 2012;13(4):1105-9. Gadgil A., Roy N., Sankaranarayanan R., Muwonge R., Sauvaget C. Effect of comprehensive breast care on breast cancer outcomes: a community hospital based study from Mumbai, India. Asian Pac J Cancer Prev. 2012;13(4):1105-9.PMID: 22799289  Gadgil A., Sauvaget C., Roy N., Grosse Frie K., Chakraborty A., Lucas E., Bantwal K., Haldar I., Sankaranarayanan R. Breast cancer awareness among middle class urban women - a community-based study from Mumbai, India. Asian Pac J Cancer Prev. 2015;16(15):6249-54. Gadgil A., Sauvaget C., Roy N., Grosse Frie K., Chakraborty A., Lucas E., Bantwal K., Haldar I., Sankaranarayanan R. Breast cancer awareness among middle class urban women - a community-based study from Mumbai, India. Asian Pac J Cancer Prev. 2015;16(15):6249-54.PMID: 26434824  Gadgil A., Sauvaget C., Roy N., Muwonge R., Kantharia S., Chakrabarty A., Bantwal K., Haldar I., Sankaranarayanan R. Cancer early detection program based on awareness and clinical breast examination: Interim results from an urban community in Mumbai, India. Breast. 2017;31:85-9. Gadgil A., Sauvaget C., Roy N., Muwonge R., Kantharia S., Chakrabarty A., Bantwal K., Haldar I., Sankaranarayanan R. Cancer early detection program based on awareness and clinical breast examination: Interim results from an urban community in Mumbai, India. Breast. 2017;31:85-9.PMID: 27829200  Gadgil A., Sauvaget C., Roy N., Muwonge R., Lucas E., Sankaranarayanan R. Setting up a Breast Cancer Awareness Project in Mumbai: Methodology, Experiences and Challenges. J Cancer Educ. 2019 Mar 12. doi: 10.1007/s13187-019-01500-x. Gadgil A., Sauvaget C., Roy N., Muwonge R., Lucas E., Sankaranarayanan R. Setting up a Breast Cancer Awareness Project in Mumbai: Methodology, Experiences and Challenges. J Cancer Educ. 2019 Mar 12. doi: 10.1007/s13187-019-01500-x.PMID: 30863980 |
| Funding: | Bhabha Atomic Research Centre (BARC) |
Legend: Ongoing projects (blue), Completed projects (green)
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85
© IARC 2026 - Terms of use - Privacy Policy.
© IARC 2026 - Terms of use - Privacy Policy.