|
Breast pathology – Fine-needle aspiration cytology (FNAC) procedure – Staining procedure |   |
The staining procedures include:
- staining to evaluate the adequacy of the aspirated material (ROSE);
- staining on wet-fixed smears with Pap or haematoxylin and eosin (H&E) stains; and
- staining on air-dried smears with any of the Romanowsky stains (e.g. Giemsa, May–Grünwald–Giemsa (MGG), Wright–Giemsa, or Diff-Quik stain).
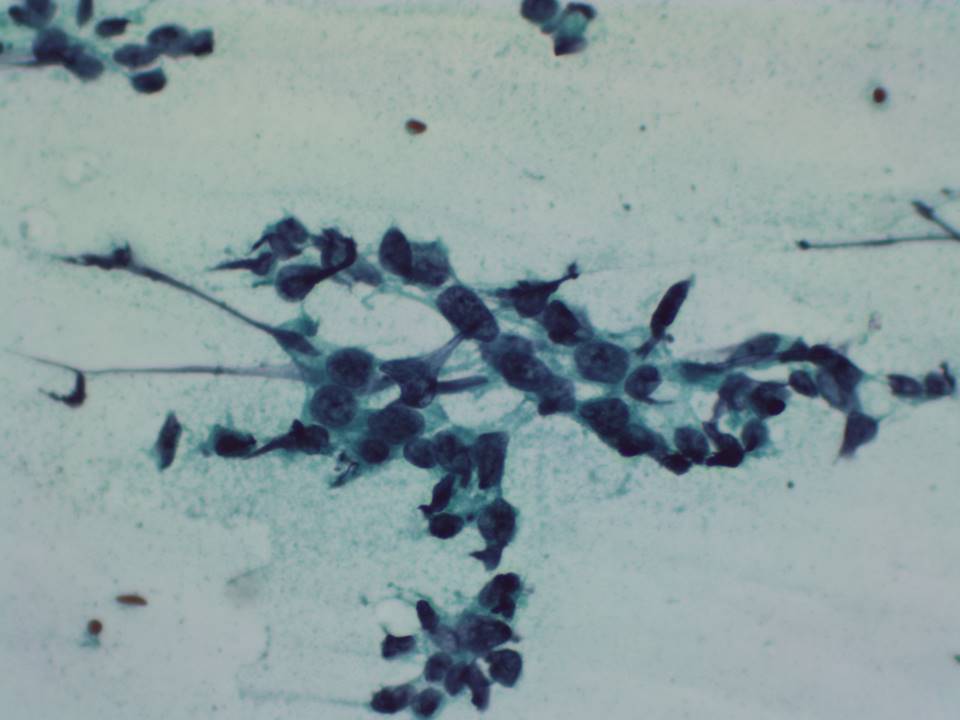
Staining for ROSE for adequacy of the aspirated material
A rapid staining technique may be used for the preliminary evaluation of material before the patient is released. It is done to ascertain that adequate material has been collected and sometimes to make a preliminary diagnosis. Toluidine blue staining is usually used for such rapid evaluation. The steps involved are as follows:
- Immerse the smear in alcohol for at least 15 seconds.
- Apply 1–2 drops of toluidine blue solution (0.05 g toluidine blue powder, 20 mL of 95% alcohol, and 80 mL of distilled water, mixed and filtered before use) to the entire smear.
- Wet-mount the smear with a coverslip.
- Allow the stain to penetrate for 10–15 seconds; then turn over the slide onto a paper towel with the coverslip in place. Remove the excess stain by applying moderate pressure to the slide.
- Observe the slide under the microscope after 1 minute.
If there is no diagnostic material, repeat the FNAC.
After microscopic evaluation, re-immerse the slide in alcohol. The coverslip will become detached and the slide can be stained by the Pap or H&E method.
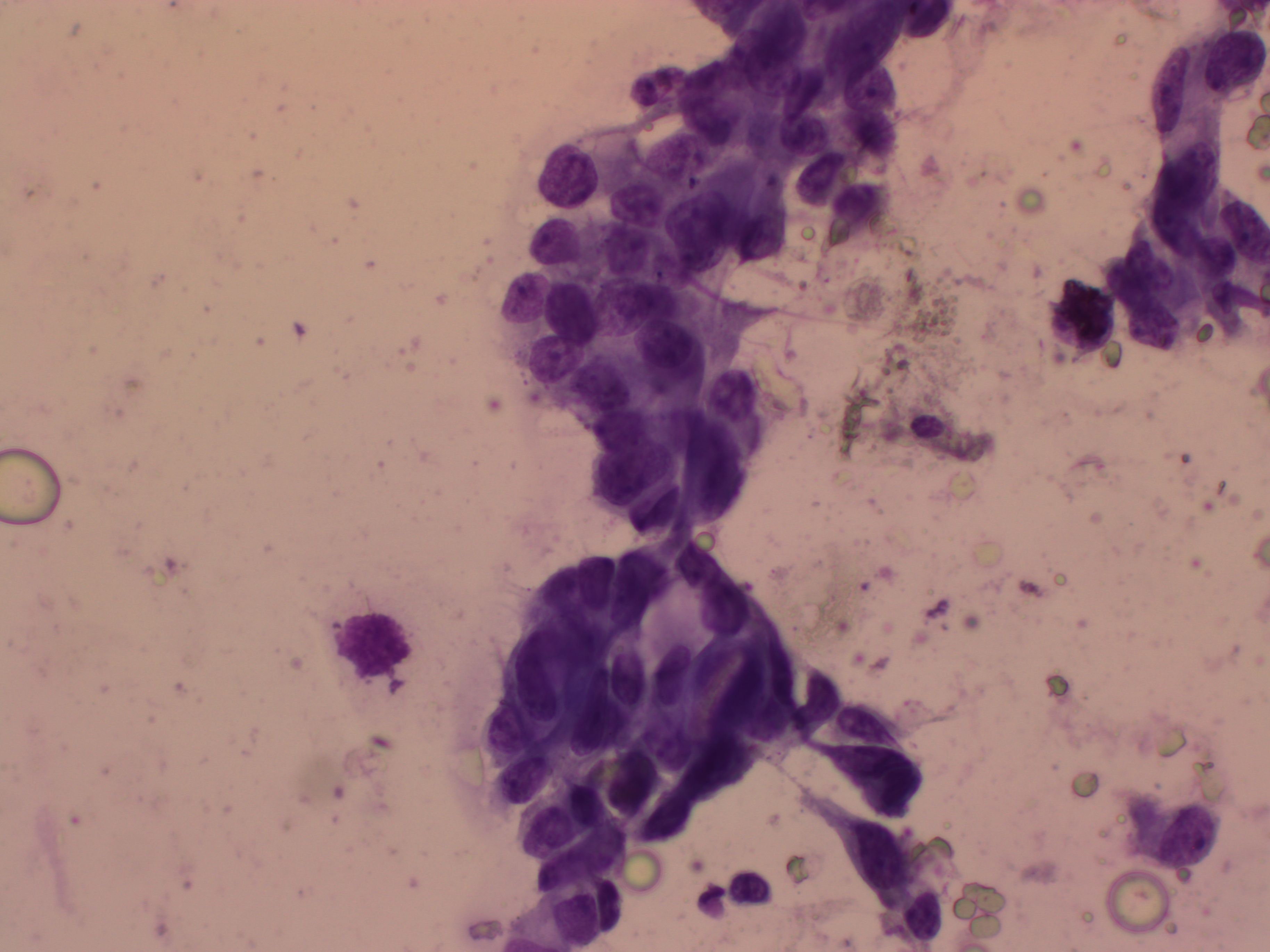
Most cytology laboratories wet-fix some smears and dry-fix the rest, so that both Pap-stained and Giemsa-stained smears are available for better differentiation of the nuclear and cytoplasmic features. H&E stain is generally not used for cytology, but is readily available in most pathology laboratories because it is the stain used for histopathology sections.
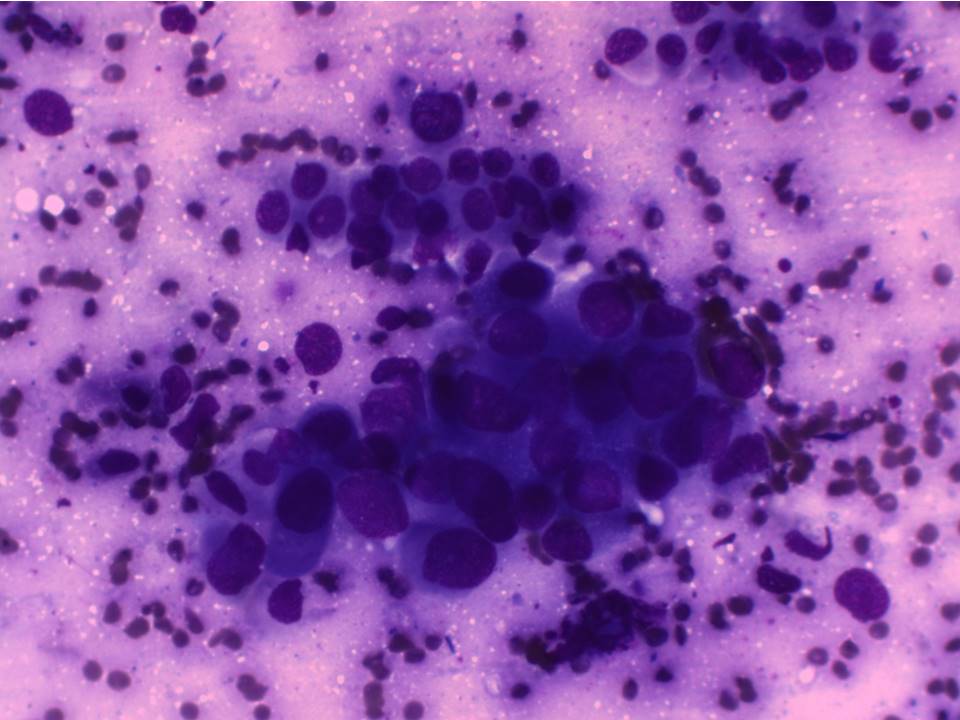
Pap staining procedure
- Transfer the slides directly from the fixative, without drying, to 95% alcohol (10 seconds).
- Place the slide in 50% alcohol (10 seconds).
- Rinse gently in running tap water (2 minutes).
- Stain in Harris haematoxylin (3 minutes).
- Rinse in running tap water (2 minutes).
- Dip in alcoholic ammonia (1 minute).
- Dehydrate by placing in 75% alcohol (15 seconds).
- Dehydrate by placing in 95% alcohol (15 seconds).
- Stain in OG 6 (2½ minutes).
- Rinse in 95% alcohol (10 seconds).
- Stain in EA 36 (30 seconds).
- Rinse in absolute alcohol (30 seconds).
- Rinse in absolute alcohol (30 seconds).
- Place slide in oven (5 minutes).
- Rinse in absolute ethanol + xylene (1:1) (2 minutes).
- Clear in xylene (5 minutes).
- Clear in xylene (5 minutes).
- Clear xylene (until totally clear).
- Mount in DPX.
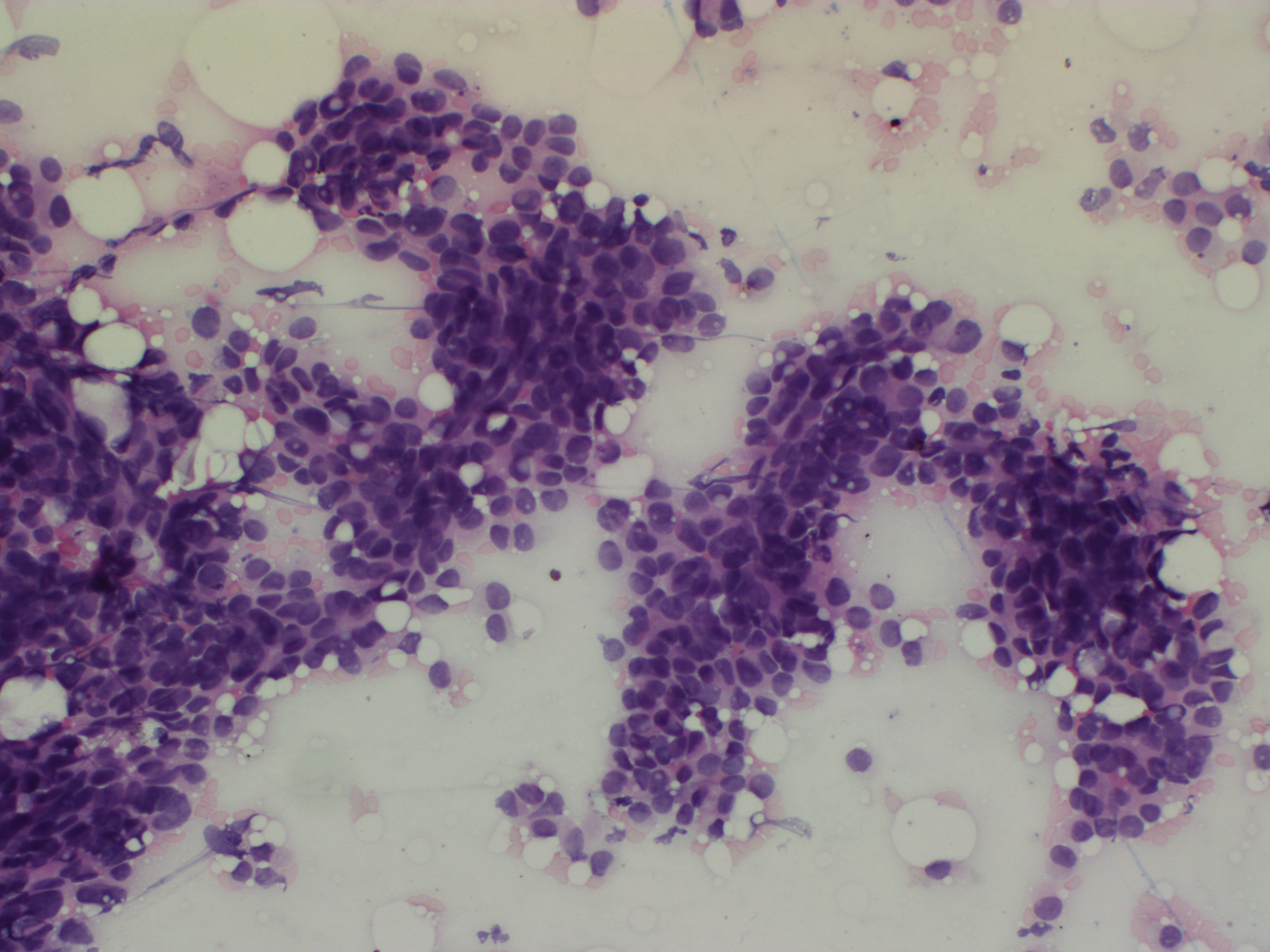
H&E staining procedure
- Transfer the slides directly from fixative, without drying, to absolute alcohol (2 minutes).
- Place in 80% alcohol (30 seconds).
- Rinse in running tap water (1½ minutes).
- Stain in haematoxylin (15 minutes).
- Rinse in running tap water (1½ minutes).
- Dip in 1% acid alcohol (2 seconds).
- Rinse in running tap water (10 minutes).
- Stain in eosin (4 minutes).
- Rinse in running tap water (1½ minutes).
- Rinse in absolute alcohol (1 minute).
- Rinse in absolute alcohol (1 minute).
- Clear in xylene (1 minute).
- Mount in DPX.
Giemsa staining procedure
- Fix the smear with methanol.
- Pour on working Giemsa stain (1:6 stock Giemsa stain diluted with buffered distilled water of pH 7.2–7.4).
- Leave for 30–35 minutes.
- Wash the slides with tap water.
- Dry and mount with DPX.
|
.png)
Click on the pictures to magnify and display the legends

Click on this icon to display a case study
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85 © IARC 2025 - Terms of use - Privacy Policy.
| .png)