|
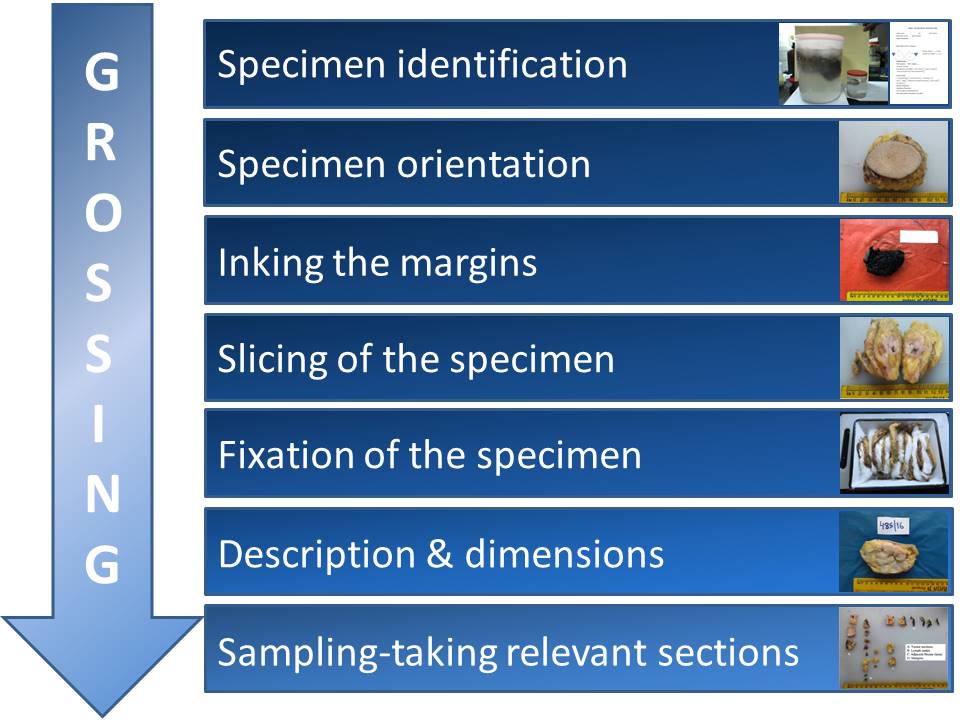
Breast pathology – Histopathology of the breast – Specimen grossing |   |
In specimen grossing, the received specimen is thoroughly inspected, measured, and described. Diagnostically valuable sections are taken as appropriate.
The objectives of grossing are:
- to identify the specimen, determine its orientation, and measure it;
- to identify the presence, location, and dimensions of any lesions (masses, calcifications, etc.);
- to estimate the distance of the lesions from the surgical margins;
- to take diagnostically valuable sections for more precise microscopic evaluation; and
- to take sections from various other locations depending on the type of specimen (e.g. nipple, all quadrants, and lymph nodes associated with mastectomies).
Preparation
Before proceeding with grossing of the specimen, it is important to:
- review the relevant clinical information;
- review the previous cytology or biopsy reports, if available;
- review the relevant radiology reports; and
- prepare the specimen diagram and obtain photos of the specimen, if possible.
Specimen identification
- Match the specimen with the patient identification and clinical details noted in the requisition form.
- Count the number of specimen(s).
- Assign a unique laboratory specimen identifier to the specimen. This is generally with a sequential numbering system starting with one (1) and proceeding to the final specimen of each year. Thus, case 57/21 is the 57th histopathology specimen of the year 2021. If more than one specimen from a single patient is received on the same day, it is numbered in this case as 57(1)/21, 57(2)/21, and so on, so that the identity of the specimen is unique.
- Note the type of specimen.
- Note the type and location of lesion(s).
- Note the date and time of collection from the patient.
- Note the date and time when placed in formalin solution. (The time to fixation from surgery, called the cold ischaemic time, should be less than 1 hour.)
Specimen orientation
- Search for the orienting stitches placed on the specimen during surgery (also review the surgeon’s note in the requisition form). Lumpectomy specimens are generally oriented with a long suture laterally and a short suture superiorly. In a modified radical mastectomy (MRM), the skin flap with nipple and areola seen anteriorly and the axillary fat with lymph nodes seen laterally help in orientation.
- Weigh the specimen and record the weight.
- Record the measurement of the whole specimen in three dimensions in centimetres.
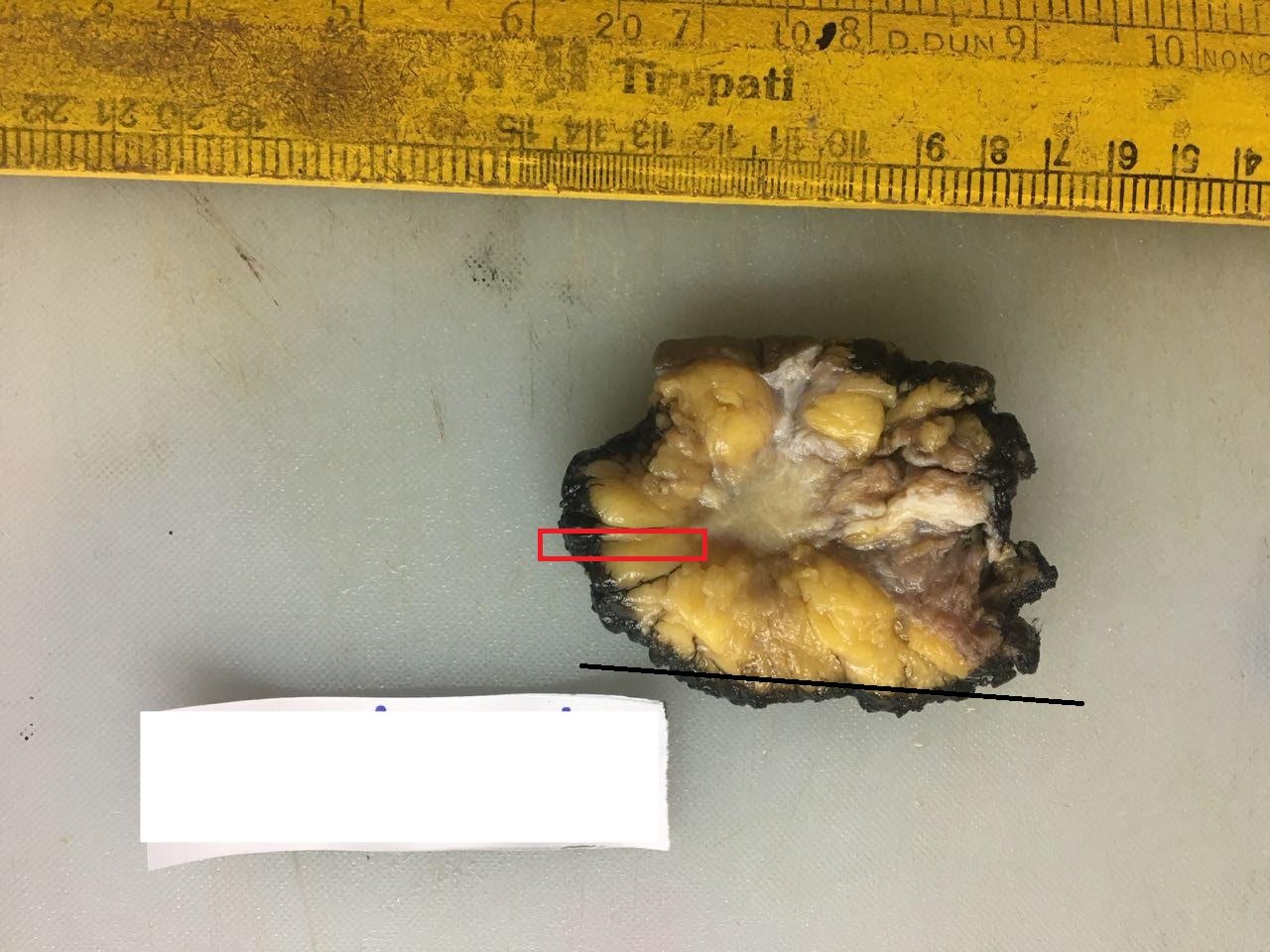
Inking the margins
The margins are inked to identify the surgical plane of excision microscopically. Any washproof ink, such as India ink or fabric paint, can be used.
- In a lumpectomy, ink the entire external surface.
- In a mastectomy specimen, only ink the entire base because the relevant surgical margin is the posterior (deep) one.
- Dry the specimen with a blotting paper first, then ink all relevant surgical margins and blot dry again.
- Apply the ink gently with a brush because forceful application can result in seepage of the ink into the specimen.
Slicing of the specimen
Specimens are sliced to facilitate fixation.
- Palpate the intact specimen.
- To locate the lesion, hold it between the thumb and forefinger to anchor it, and then make a slice through the centre of the lesion to expose the lesion completely.
- Then serially section the specimen to demonstrate the closest margin radially.
- Mastectomy specimens should be serially sectioned perpendicular to the skin at 1 cm intervals from the posterior aspect of the excised breast.
Fixation of the specimen
- Use 10% neutral-buffered formalin as the fixative; pH should be maintained at 7.2–7.4.
- After slicing, immerse the specimen in the fixative as soon as possible to allow for adequate penetration of the fixative.
- Sliced MRM specimens should be packed with gauze, and fixed in formalin overnight.
- The recommended fixation time is a minimum of 6 hours and a maximum of 72 hours.
- According to the current College of American Pathologists/American Society of Clinical Oncology guidelines, even small specimens such as core needle biopsy samples should be fixed in formalin for 6–48 hours.
- Underfixation of breast tissue may lead to false-negative ER results and overfixation may lead to false-positive HER2 results on IHC.
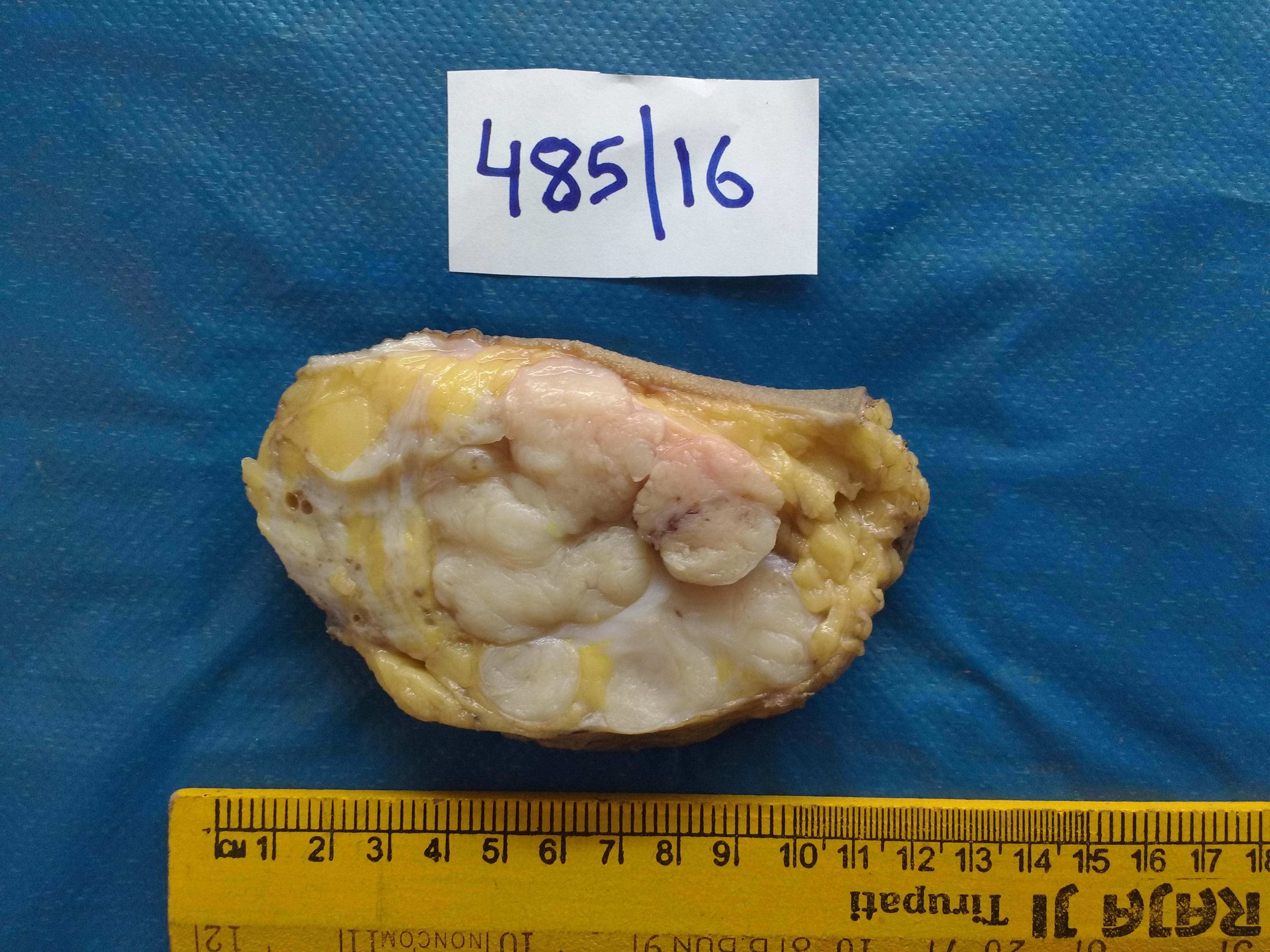
Measuring the dimensions of the specimen
- For core needle biopsy, record the number of cores and length of each core in millimetres.
- Use specimen diagrams, structured checklists, and standard templates to note down the details of the specimen.
- Note the tumour size in three dimensions and the minimum distance from the margin.
- Describe the consistency and note the presence of necrosis, fibrosis, cysts (number, size, content), and calcifications.
- Describe any gross abnormalities of skin and nipple or the presence of any scar or earlier biopsy site or cavity.
- Note whether the margins are submitted as shaved or radial (perpendicular).
Grossing of the axillary lymph nodes
- Identify all the lymph nodes and count them.
- Identify gross metastases to the nodes if present, record the number, measure, and submit just one representative section from each to document involvement. Grossly uninvolved nodes should be thinly sliced at 2 mm along their long axis and all embedded. Nodes about 5 mm should be bisected and embedded. Nodes < 5 mm should be embedded whole.
- Do not include more than one sectioned lymph node in each cassette to avoid error in counting the lymph nodes.
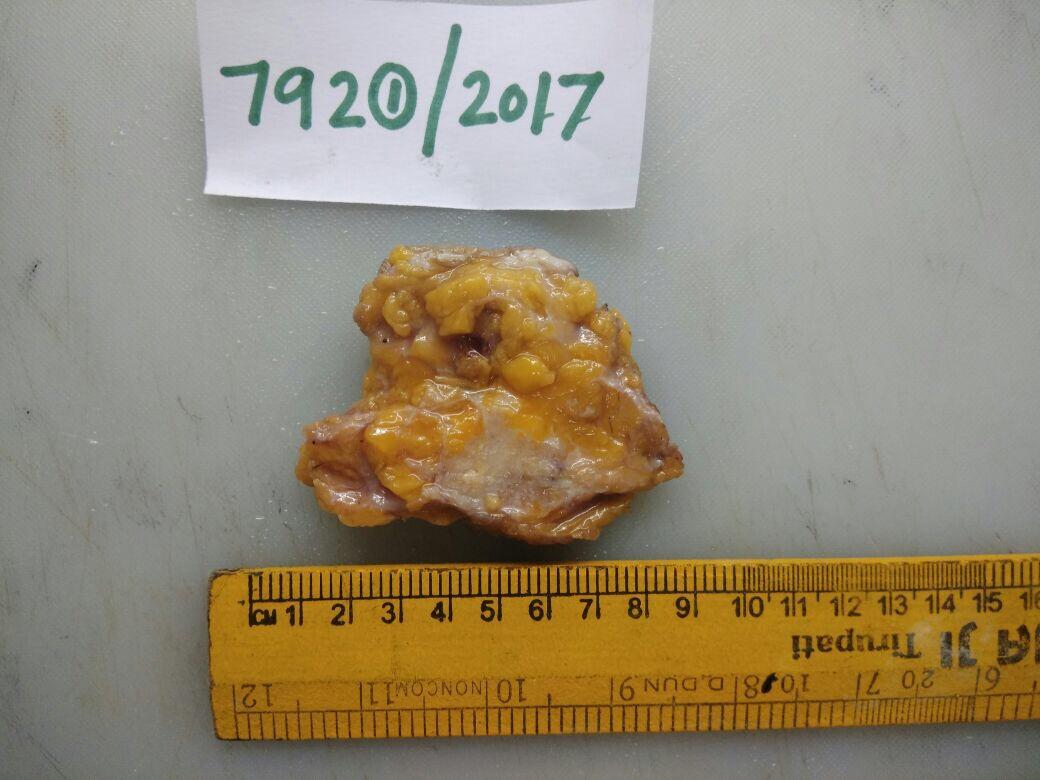
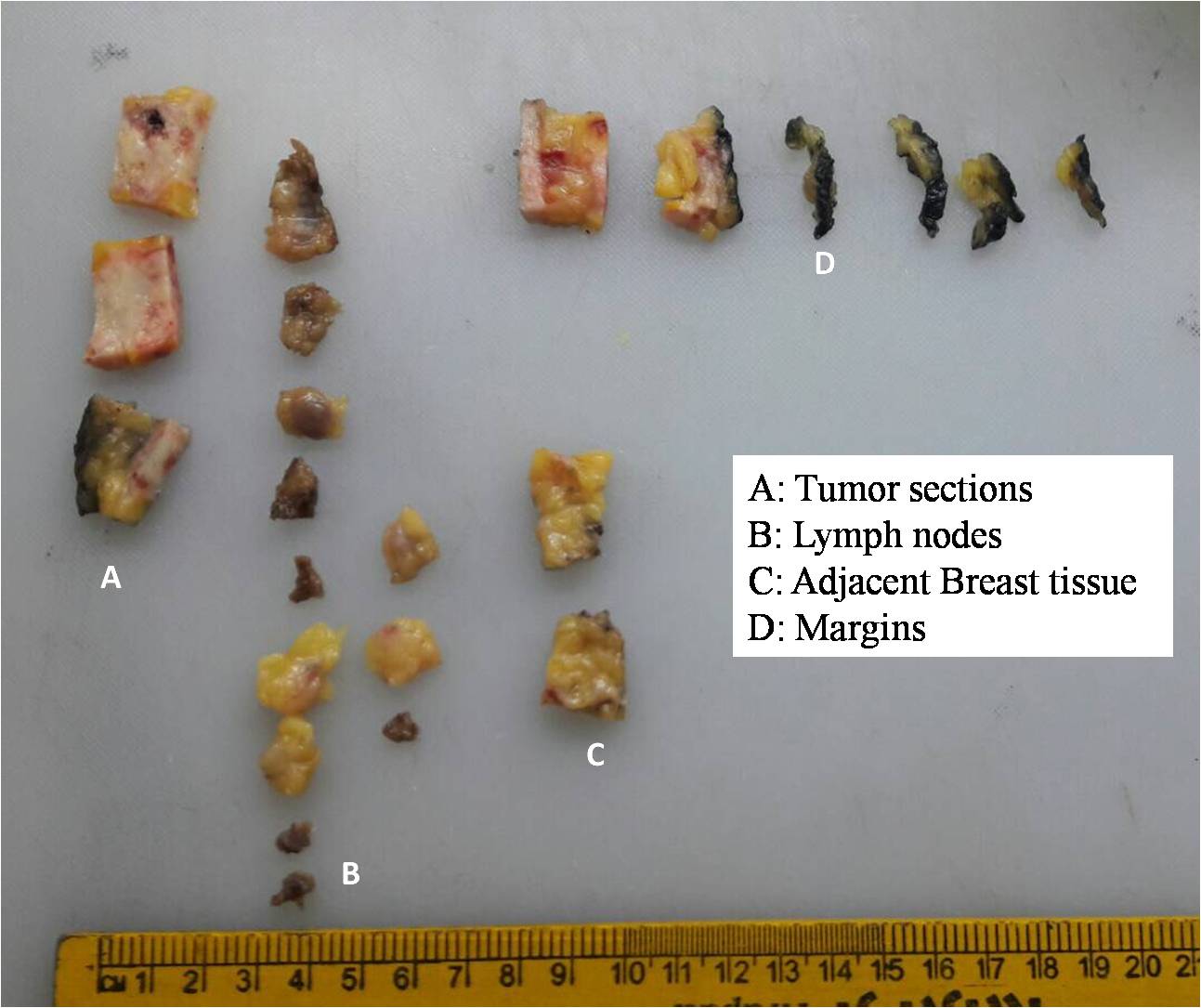
Sampling
- Appropriate sections should be taken from all specimens received so that diagnostically valuable information can be obtained from them.
- Because every section taken is further processed into a paraffin block, it is important to maintain the unique identification throughout the process. Hence, the sections should be submitted in a suitable definite protocol along with a diagram to show where the sections were taken from.
- Generally, subparts of the specimen are designated alphabetically, as tumour area (A), nipple/skin (B), cut margin (C), uninvolved breast tissue (D), lymph nodes (E), frozen section bit (FS), and counterpart of the frozen section (CP).
- If there are multiple sections of the above-mentioned subparts, then sequential numbers are added to the designated alphabet letter. Hence, if four sections are given from the tumour area, they would be labelled as A1, A2, A3, and A4. Likewise, axillary nodes would be labelled as E1, E2, E3, and so on.
- All sections taken are then placed on the grossing board with their respective labels.
- The pathologist should draw a diagram of each section on the white paper attached to the pathology form. The technologist should countercheck the number of sections with the diagram before putting them in the cassettes with the corresponding label.
- The technologist then notes down the number of cassettes prepared for that particular specimen.
- The cassettes should be placed in the formalin fixative jars maintained in the grossing room for processing.
- Very tiny sections should be wrapped in filter paper (e.g. Whatman no. 1) labelled with the corresponding pathology number and placed in cassettes to prevent loss of tissue during processing.
- Tissue sections should not be oversized or stuffed in cassettes. The size of the tissue sections should not be very big nor should many tissue sections be stuffed in one cassette. This will ensure easy flow of reagents into the cassette onto the tissue section during processing.
- The thickness of the tissue should be uniform and no more than 2–3 mm.
- Colour-coded cassettes should be used to differentiate the different types of tissues to be processed.
The sections to be submitted after grossing of a radical mastectomy specimen
After grossing of a radical mastectomy specimen, the following sections should be submitted:
- four sections of tumour with adjacent breast parenchyma;
- nipple and areola;
- skin overlying the tumour;
- deep margin of the base;
- any suspicious grey-white area;
- axillary lymph nodes; and
- level 3 lymph nodes from the apex whenever available.
The sections to be submitted after grossing of a breast-conserving surgery specimen
After grossing of a specimen from breast-conserving surgery, the following sections should be submitted:
- sections of tumour with adjacent breast;
- skin or nipple disc if present;
- section of breast from uninvolved areas; and
- margins: superior, inferior, medial, lateral, anterior (skin if present), posterior axillary nodes.
|   |
|
.png)
Click on the pictures to magnify and display the legends

Click on this icon to display a case study
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85 © IARC 2026 - Terms of use - Privacy Policy.
| .png)