Image | Caption |
 | Figure 1: Schematic representation of the steps in diagnosis of oral leukoplakia adapted from Warnakulasuriya et al 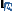 . . |
 | Table 2: Criteria used for diagnosing dysplasia extracted from Blue Book (Head and Neck tumours (p177) 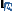 ). ). |
 | Figure 2: Leukoplakia with mild dysplasia. Hyper keratotic, with hyperplastic epithelium with elongated rete pegs and abnormal cells in the lower one-third of the epithelium. |
 | Figure 3: Leukoplakia with moderate dysplasia. Hyper keratotic, with hyperplastic squamous epithelium with abnormal cells in two-thirds the thickness of epithelium. |
 | Figure 4: Leukoplakia with severe dysplasia. Hyperplastic, with hyperplastic squamous epithelium with markedly abnormal cells in more than two-thirds of the epithelium. |