Home / Reseach Projects / Oral cancer early detection
Legend: Ongoing projects (blue), Completed projects (green)
Research projects
Oral cancer early detection
| Study sites: | India: Ambilikkai, Bangalore, Barshi, Guwahati, Sevagram, Thiruvananthapuram |
| Principal investigator (PI) from IARC: | M. Tommasino |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2011 |
| Closure date: | Ongoing |
| Objectives: | Overall objective
|
| Methodology: | This is a multicentre study including recruiting centres in Europe and India. The Screening Group is collaborating with the Indian centres. Approximately 4000–6000 HNC specimens are collected from several Indian centres from rural or urban areas. The specimens include cancers of the hypopharynx, oropharynx, oral cavity, and larynx. Each Indian centre provides approximately 1000–1500 specimens representing the four anatomical sites. Epidemiological and clinical data are also collected when available. | Publications: | Simoens C., Gorbaslieva I., Gheit T., Holzinger D., Lucas E., Ridder R., Rehm S., Vermeulen P., Lammens M., Vanderveken O.M., Kumar R.V., Gangane N., Caniglia A., Maffini F., Rubio M.B.L. , Anantharaman D., Chiocca S., Brennan P., Pillai M.R., Sankaranarayanan R., Bogers J., Pawlita M., Tommasino M., Arbyn M., HPV-AHEAD study group. HPV DNA genotyping, HPV E6*I mRNA detection, and p16 INK4a/Ki-67 staining in Belgian head and neck cancer patient specimens, collected within the HPV-AHEAD study. Cancer Epidemiol. 2021 Apr 8;72:101925. PMID: 33839457 Ursu R.G., Danciu M., Spiridon I.A., Ridder R., Rehm S., Maffini F., McKay-Chopin S., Carreira C., Lucas E., Costan V.V., Popescu E., Cobzeanu B., Ghetu N., Iancu L.S., Tommasino M., Pawlita M., Holzinger D., Gheit T. Role of mucosal high-risk human papillomavirus types in head and neck cancers in Romania. PLoS One. 2018 Jun 25;13(6):e0199663. PMID: 29940024 Gheit T., Anantharaman D., Holzinger D., Alemany L., Tous S., Lucas E., Prabhu P.R., Pawlita M., Ridder R., Rehm S., Bogers J., Maffini F., Chiocca S., Lloveras B., Kumar R.V., Somanathan T., de Sanjosé S., Castellsagué X., Arbyn M., Brennan P., Sankaranarayanan R., Pillai M.R., Gangane N., Tommasino M.; HPV-AHEAD study group. Role of mucosal high-risk human papillomavirus types in head and neck cancers in central India. Int J Cancer. 2017 Jul 1;141(1):143-151. PMID: 28369859 Mena M., Lloveras B., Tous S., Bogers J., Maffini F., Gangane N., Kumar R.V., Somanathan T., Lucas E., Anantharaman D., Gheit T., Castellsagué X., Pawlita M., de Sanjosé S., Alemany L., Tommasino M.; HPV-AHEAD study group. Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: The example from the HPV-AHEAD study. PLoS One. 2017 Oct 16;12(10) PMID: 29036167 |
| Funding: | European Union |
| Study sites: | Thiruvananthapuram (formerly called Trivandrum), Kerala, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | K. Ramadas (PI), S. Thara, Beela Sarah Mathew, Elizabeth Abraham, Gigi Thomas, Jem Prabhakar, Paul Augustine, Ramani Wesley, R. Rejnish Kumar, Regional Cancer Centre (RCC), Thiruvananthapuram, Kerala, India |
| Map: |  |
| Start date: | 1995 |
| Closure date: | 2013 |
| Objectives: |
|
| Methodology: | 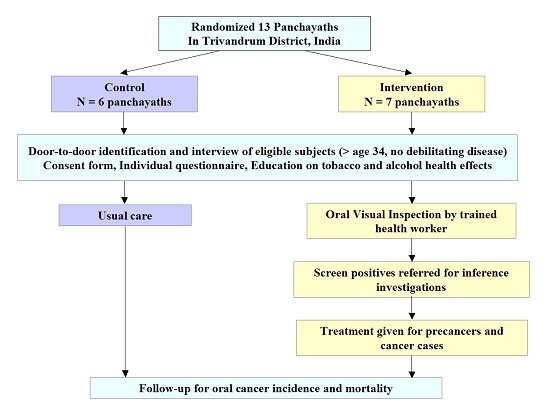 |
| Study outcomes: | In 1996–2004, three rounds of oral visual inspection were provided by trained health workers at 3-year intervals to eligible individuals (aged ≥ 34 years) in the intervention group. We reported a 35% decrease in mortality rate among the tobacco users/alcohol consumers of the intervention group compared with the control group. This evidence was used by India’s National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS) to support their recommendation of oral visual screening of the population. | Publications: | Cheung L.C., Ramadas K., Muwonge R., Katki H.A., Thomas G., Graubard B.I., Basu P., Sankaranarayanan R., Somanathan T., Chaturvedi A.K. Risk-Based Selection of Individuals for Oral Cancer Screening. J Clin Oncol. 2021 Jan 15:JCO2002855. PMID: 33449824 Sankaranarayanan R., Ramadas K., Thara S., Muwonge R., Thomas G., Anju G., Mathew B. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013;49(4):314-21. PMID: 23265945 Ramadas K., Sauvaget C., Thomas G., Fayette J.M., Thara S., Sankaranarayanan R. Effect of tobacco chewing, tobacco smoking and alcohol on all-cause and cancer mortality: a cohort study from Trivandrum, India. Cancer Epidemiol. 2010;34(4):405-12. PMID: 20444665 Subramanian S., Sankaranarayanan R., Bapat B., Somanathan T., Thomas G., Mathew B., Vinoda J., Ramadas K. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bull World Health Organ. 2009;87(3):200-6. PMID: 19377716 Sankaranarayanan R., Ramadas K., Thomas G., Muwonge R., Thara S., Mathew B., Rajan B.; Trivandrum Oral Cancer Screening Study Group. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927-33. PMID: 15936419 Ramadas K., Sankaranarayanan R., Jacob B.J., Thomas G., Somanathan T., Mahé C., Pandey M., Abraham E., Najeeb S., Mathew B., Parkin D.M., Nair M.K. Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncol. 2003 Sep;39(6):580-8. PMID: 12798401 |
| Funding: |
| Media: |
Legend: Ongoing projects (blue), Completed projects (green)
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85
© IARC 2024 - Terms of use - Privacy Policy.
© IARC 2024 - Terms of use - Privacy Policy.



