Image | Caption |
 | Figure 1: Early oral submucous fibrosis. Hyperkeratotic minimally hyperplastic epithelium with inflammatory cells and congested capillaries in an edematous sub epithelial connective tissue. |
 | Figure 2: Advanced oral submucous fibrosis. Atrophic squamous epithelium with collagenisation of the sub epithelial tissue with scanty inflammatory cells. |
 | Figure 3: Advanced oral submucous fibrosis. Atrophic squamous epithelium with collaginisation of the sub epithelial tissue with scanty inflammatory cells. |
 | Figure 4: Advanced oral submucous fibrosis with dysplastic changes. Atrophic squamous epithelium with collagenisation of the sub epithelial tissue with scanty inflammatory cells. |
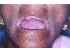 | Figure 5: Restricted mouth opening in a patient with oral submucous fibrosis (OSF). Note the blanching and extensive depapillation of the tongue. |
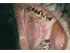 | Figure 6: Oral submucous fibrosis. Note the diffuse blanching on the right buccal mucosa, with severe blanching in the retromolar area. Note tobacco stains on the tongue and teeth. |
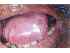 | Figure 7: Oral submucous fibrosis of the tongue. Note the coexisting verrucous leukoplakia. |
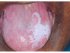 | Figure 8: Oral submucous fibrosis. Note the coexisting homogeneous leukoplakia on the left side of dorsum tongue. |
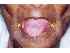 | Figure 9: Oral submucous fibrosis. Note the extensive depapillation and difficulty in protruding the tongue. Note the associated angular cheilitis (yellow arrows). |
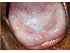 | Figure 10: Oral submucous fibrosis with coexisting homogeneous leukoplakia dorsum tongue. Note the discrete white patch and total depapillation of the dorsum of tongue and the limitation in its protrusion.The tongue has a smooth and shining appearance. |
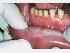 | Figure 11: Oral submucous fibrosis. Note the blanching on the lower labial mucosa. |
 | Figure 12: Note the diffuse blanching (marble-like appearance) on the right buccal mucosa of a habitual betel quid chewer. The betel quid stains can be appreciated on the lingual aspect of maxillary teeth, which have undergone attrition. |
 | Figure 13: Oral submucous fibrosis. Note the multiple pinpoint petechiae and generalised depapillation of the tongue. |
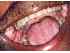 | Figure 14: Localized oral submucous fibrosis. Note the restricted mouth opening and the normal tongue in this 58–year-old habitual areca nut chewer with oral submucous fibrosis. |
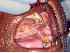 | Figure 15: Oral submucous fibrosis. Photograph of the same patient showing the hard and soft palate, and the left buccal mucosa. Note the intermingling of the hypo- and hyperpigmented areas which are seen typically in areca nut chewers. |
 | Figure 16: Regeneration of the papillae in a 65–year-old lady who started chewing at the age of 7 and stopped the habit when she was 47 years. |
 | Figure 17: Tongue in an oral submucous fibrosis patient. Note the regeneration of papillae in the central part and tip of tongue in this 76–year-old female patient who chewed betel quid from age 18 to 56. |
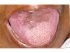 | Figure 18: Oral submucous fibrosis. Note the regeneration of papillae in the anterior part of the tongue (red arrows), three and half years after cessation of habits. Note also the hyperpigmentation (yellow arrows) in the mid part of dorsum tongue. |
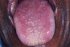 | Figure 19: Oral submucous fibrosis. Note the regeneration of papillae on the tip and mid-part of dorsum tongue 6 years after cessation of habits. |