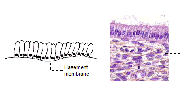Home / Training / Manuals / A practical manual on visual screening for cervical neoplasia / Anatomical and pathological basis of visual inspection
 /
/ 
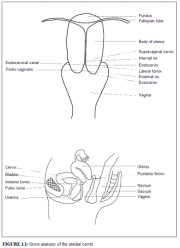
fig 1.1: Gross anatomy of the uterine cervix
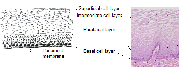
fig 1.2: Stratified squamous epithelium (x20)
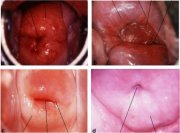
fig 1.4: Location of squamocolumnar junction (SCJ)
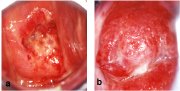
fig 1.7: A schematic diagram of further maturation of immature squamous metaplasia.

table 1: Correlation between CIN, dysplasia and the Bethesda terminology.
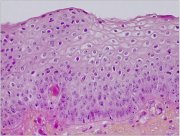
fig 1.8: Histology of CIN 1: The dyplastic cells are confined to the lower third of the epithelium (x20)
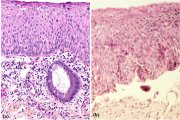
fig 1.9: Histology of CIN 2: Atypical cells are found mostly in the lower two-thirds of the epithelium (x10)
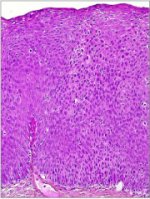
fig 1.10: Histology of CIN 3: Dysplastic cells are distributed in the full thickness of the epithelium with loss of polarity of cells (x20)
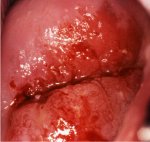
fig 1.11: Early invasive cervical cancer: note the irregular, granular, nodular surface with bleeding on touch.
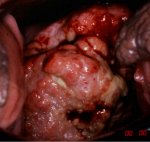
fig 1.12: Advanced invasive cervical cancer: note the bulging, cauliflower-like, ulceroproliferative growth with bleeding and necrosis.
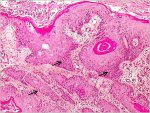
fig 1.13: Histology - Keratinizing well differentiated invasive squamous cell carcinoma. Note the stroma is infiltrated by sheets of malignant cells (x10)
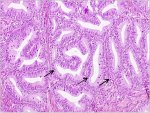
fig 1.14: Histology - Well differentiated invasive adenocarcinoma. Note the malignant cells lining the cervical crypts (x20)
A practical manual on visual screening for cervical neoplasia
Chapter 1 / Anatomical and pathological basis of visual inspection with acetic acid (VIA) and with Lugolís iodine (VILI)
Filter by language: English / FranÁais / EspaŮol / Turkish / /
/ 
Introduction
Naked-eye visual inspection of the uterine cervix, after application of 5% acetic acid (VIA) and/or of Lugolís iodine (VILI), provides simple tests for the early detection of cervical precancerous lesions and early invasive cancer. VILI is similar to the Schillerís iodine test, which was used for early detection of cervical neoplasia in the third and fourth decades of the 20th century, but discontinued after the advent of cervical cytology testing. The potential difficulties in implementing cervical cytology-based screening in low-resource settings have prompted the investigation of the accuracy of alternative low-technology tests such as VIA and VILI in the early detection of cervical neoplasia.
The results of VIA and VILI are immediately available and do not require any laboratory support. The categorization of the results of VIA or VILI depends upon the colour changes observed on the cervix. A clear understanding of the anatomy, physiology and pathology of the cervix is absolutely essential to understand the basis and to interpret the outcome of screening using VIA and VILI. The objective of this manual is to help a range of health-care providers such as doctors, nurses, midwives and health workers to acquire the skills and competence in administering and reporting the results of these tests by describing their basis and practice.
The results of VIA and VILI are immediately available and do not require any laboratory support. The categorization of the results of VIA or VILI depends upon the colour changes observed on the cervix. A clear understanding of the anatomy, physiology and pathology of the cervix is absolutely essential to understand the basis and to interpret the outcome of screening using VIA and VILI. The objective of this manual is to help a range of health-care providers such as doctors, nurses, midwives and health workers to acquire the skills and competence in administering and reporting the results of these tests by describing their basis and practice.
Gross anatomy of the uterine cervix
The cervix, constituting the lower portion of the uterus, is cylindrical or conical in shape, and measures 3 - 4 cm in length and 2.5 - 3.5 cm in diameter. It varies in size and shape depending on the age, parity and hormonal status of the woman. The lower half of the cervix, called portio vaginalis, protrudes into the vagina through its anterior wall, and the upper half, called the supravaginal portion, remains above the vagina (figure 1.1). The cervix opens into the vagina through the external os. The supravaginal portion meets the body of the uterus at the internal os. In parous women, the cervix is bulky and the external os appears as a wide, gaping, transverse slit. In nulliparous women, the external os resembles a small circular (pinhole) opening.
The portion of the cervix that is exterior to the external os is called the ectocervix, which is readily visible during speculum examination. The portion above the external os is called the endocervix. The endocervical canal, which traverses the endocervix, connects the uterine cavity with the vagina and extends from the internal to the external os. The portion of the upper vaginal cavity that surrounds the portio vaginalis is called the fornix.
The stroma of the cervix is composed of dense, fibro-muscular tissue traversed by the vascular, lymphatic and nerve supplies to the cervix. The arteries of the cervix, derived from internal iliac arteries through the cervical and vaginal branches of the uterine arteries, descend in the lateral aspects of the cervix at 3 and 9 o'clock positions. The veins run parallel to the arteries and drain into the hypogastric venous plexus. The lymphatic vessels from the cervix drain into the common, internal and external iliac nodes, obturator and the parametrial nodes. The nerve supply is derived from the hypogastric plexus. The endocervix has extensive sensory nerve endings, while there are very few in the ectocervix. Hence, procedures such as biopsy and cryotherapy are well tolerated in most women, without local anaesthesia. Since sympathetic and parasympathetic fibres are also abundant in the endocervix, manipulation of the endocervix may stimulate these nerve endings, occasionally leading to giddiness or fainting attacks.
The portion of the cervix that is exterior to the external os is called the ectocervix, which is readily visible during speculum examination. The portion above the external os is called the endocervix. The endocervical canal, which traverses the endocervix, connects the uterine cavity with the vagina and extends from the internal to the external os. The portion of the upper vaginal cavity that surrounds the portio vaginalis is called the fornix.
The stroma of the cervix is composed of dense, fibro-muscular tissue traversed by the vascular, lymphatic and nerve supplies to the cervix. The arteries of the cervix, derived from internal iliac arteries through the cervical and vaginal branches of the uterine arteries, descend in the lateral aspects of the cervix at 3 and 9 o'clock positions. The veins run parallel to the arteries and drain into the hypogastric venous plexus. The lymphatic vessels from the cervix drain into the common, internal and external iliac nodes, obturator and the parametrial nodes. The nerve supply is derived from the hypogastric plexus. The endocervix has extensive sensory nerve endings, while there are very few in the ectocervix. Hence, procedures such as biopsy and cryotherapy are well tolerated in most women, without local anaesthesia. Since sympathetic and parasympathetic fibres are also abundant in the endocervix, manipulation of the endocervix may stimulate these nerve endings, occasionally leading to giddiness or fainting attacks.

fig 1.1: Gross anatomy of the uterine cervix
Microscopic anatomy
Squamous epithelium
The cervix is covered by two types of epithelium, stratified squamous epithelium and columnar epithelium, which meet at the squamocolumnar junction. A large area of ectocervix is covered by the stratified, non-keratinizing, glycogen-containing squamous epithelium. It is opaque, has multiple (15-20) layers of cells (figure 1.2) and appears pale pink in colour on visual examination. It consists of a single layer of round basal cells with a large dark-staining nucleus and little cytoplasm at the basement membrane. The basement membrane separates the epithelium from the underlying stroma. The basal cells divide and differentiate to form the parabasal, intermediate and superficial layers. From the basal to the superficial layer, the cells undergo an increase in cytoplasm and a reduction of nuclear size. The intermediate and superficial layer cells contain abundant glycogen in their cytoplasm. Since iodine readily stains with glycogen, application of Lugol's iodine on the squamous epithelium will result in mahogany brown or black coloration. In postmenopausal women, the cells in the squamous epithelium do not mature beyond the parabasal layer, and do not accumulate as multiple layers of intermediate and superficial cells. Consequently, the squamous epithelium becomes thin and atrophic. Thus, it appears pale and brittle, with sub-epithelial petechiae, as it is easily prone to trauma.
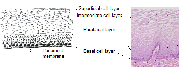
fig 1.2: Stratified squamous epithelium (x20)
Columnar epithelium
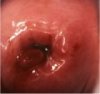
The endocervical canal is lined by the columnar epithelium (sometimes referred to as glandular epithelium), composed of a single layer of tall cells with dark-staining nuclei (figure 1.3). On visual examination, it appears as a grainy, strikingly reddish area because the thin single cell layer allows the coloration of the underlying stroma to be seen more easily. It forms several invaginations into the substance of the cervical stroma, resulting in the formation of endocervical crypts (sometimes referred to as endocervical glands). The columnar cells secrete the mucus that lubricates the cervix and vagina. At its upper limit, it merges with the endometrial epithelium in the body of uterus, and at its lower limit, it meets with the squamous epithelium at the squamocolumnar junction. A localized proliferation of the columnar epithelium in the form of a polyp may occasionally be visible as a reddish mass protruding from the external os (figure 2.2). As columnar epithelium does not produce glycogen, it does not change colour after the application of Lugol's iodine, or remains slightly discoloured with a thin film of iodine solution.
Squamocolumnar junction
The squamocolumnar junction (figure 1.4) appears as a sharp line. The location of the squamocolumnar junction in relation to the external os varies, depending upon factors such as age, hormonal status, birth trauma and certain physiological conditions such as pregnancy (figure 1.4). During childhood and perimenarche, it is located at, or very close to, the external os. After puberty and during the reproductive period, the female genital organs grow under the influence of estrogen. Thus, the cervix enlarges and the endocervical canal elongates. This leads to the eversion of the columnar epithelium onto the ectocervix, particularly on the anterior and posterior lips, resulting in ectropion or ectopy. Thus, the squamocolumnar junction is located in the ectocervix, far away from the external os during the reproductive years and pregnancy (figure 1.4a). On visual inspection, ectropion is seen as a strikingly reddish ectocervix (figure 1.4a).
The buffer action of the mucus covering the columnar cells is interfered with when the everted columnar epithelium is exposed to the acidic vaginal environment. This leads to the destruction and eventual replacement of the columnar epithelium by the newly formed metaplastic squamous epithelium. Metaplasia refers to the change or replacement of one type of epithelium by another. As a woman passes through her reproductive life to the perimenopausal age group, the location of the squamocolumnar junction progressively starts moving on the ectocervix towards the external os (figures 1.4b and c). Thus, it is located at variable distances from the external os, as a result of the progressive formation of the new metaplastic squamous epithelium in the exposed areas of the columnar epithelium in the ectocervix. From the perimenopausal period and after the onset of menopause, the cervix shrinks, due to the lack of estrogen, and, consequently, the movement of the squamocolumnar junction towards the external os and into the endocervical canal is further accelerated (figure 1.4c). In postmenopausal women, the squamocolumnar junction is located in the endocervical canal and, hence, often cannot be seen on visual examination (figure 1.4d).
The buffer action of the mucus covering the columnar cells is interfered with when the everted columnar epithelium is exposed to the acidic vaginal environment. This leads to the destruction and eventual replacement of the columnar epithelium by the newly formed metaplastic squamous epithelium. Metaplasia refers to the change or replacement of one type of epithelium by another. As a woman passes through her reproductive life to the perimenopausal age group, the location of the squamocolumnar junction progressively starts moving on the ectocervix towards the external os (figures 1.4b and c). Thus, it is located at variable distances from the external os, as a result of the progressive formation of the new metaplastic squamous epithelium in the exposed areas of the columnar epithelium in the ectocervix. From the perimenopausal period and after the onset of menopause, the cervix shrinks, due to the lack of estrogen, and, consequently, the movement of the squamocolumnar junction towards the external os and into the endocervical canal is further accelerated (figure 1.4c). In postmenopausal women, the squamocolumnar junction is located in the endocervical canal and, hence, often cannot be seen on visual examination (figure 1.4d).

fig 1.4: Location of squamocolumnar junction (SCJ)
Squamous metaplasia
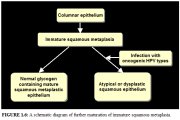
The earliest event in squamous metaplasia is the appearance of small, round, sub-columnar cells in the exposed areas of the columnar epithelium, called reserve cells (figure 1.5a). These reserve cells proliferate (figure 1.5b) and differentiate to form a thin, non-stratified, multicellular epithelium called immature squamous epithelium (figure 1.5c). The cells in the immature squamous metaplastic epithelium do not produce glycogen and, hence, do not stain brown or black with Lugol's iodine solution. Numerous foci of immature squamous metaplasia may arise at the same time.
Further development of the newly formed immature metaplastic epithelium may take either one of two directions (figure 1.6). In the vast majority of women, it develops into a mature, stratified, glycogen-producing, squamous metaplastic epithelium, which is similar to the squamous epithelium found on the ectocervix, for all practical purposes (figure 1.5d). Thus, it stains brown or black after the application of Lugol's iodine. Several cysts, called nabothian cysts, may be observed in the mature metaplastic squamous epithelium (figure 2.3). These are retention cysts that develop as a result of the occlusion of the crypt openings in the trapped columnar epithelium by the overlying metaplastic squamous epithelium. The buried columnar epithelium in the cysts may continue to secrete mucus, which eventually distends the cysts. The entrapped mucus gives an ivory-white hue to the cyst on visual examination.
In a very small minority of women, the immature squamous metaplasia may turn into a dysplastic epithelium (an altered epithelium showing precancerous cellular changes), due to infection with certain human papillomavirus (HPV) types (figure 1.6).
Further development of the newly formed immature metaplastic epithelium may take either one of two directions (figure 1.6). In the vast majority of women, it develops into a mature, stratified, glycogen-producing, squamous metaplastic epithelium, which is similar to the squamous epithelium found on the ectocervix, for all practical purposes (figure 1.5d). Thus, it stains brown or black after the application of Lugol's iodine. Several cysts, called nabothian cysts, may be observed in the mature metaplastic squamous epithelium (figure 2.3). These are retention cysts that develop as a result of the occlusion of the crypt openings in the trapped columnar epithelium by the overlying metaplastic squamous epithelium. The buried columnar epithelium in the cysts may continue to secrete mucus, which eventually distends the cysts. The entrapped mucus gives an ivory-white hue to the cyst on visual examination.
In a very small minority of women, the immature squamous metaplasia may turn into a dysplastic epithelium (an altered epithelium showing precancerous cellular changes), due to infection with certain human papillomavirus (HPV) types (figure 1.6).
Transformation zone
The transformation zone is the area of the cervix where the columnar epithelium has been replaced and/or is being replaced by the metaplastic squamous epithelium. With the naked eye, one can identify the inner border of the transformation zone by tracing the squamocolumnar junction and the outer border by locating the distal most nabothian cysts (if present) or crypt openings (usually visible under magnification). In premenopausal women, the transformation zone is primarily located on the ectocervix. After menopause, and through old age, the cervix shrinks with the decreasing levels of oestrogens. Consequently, the transformation zone may move partially, and later fully, into the endocervical canal. Almost all cervical neoplasia occurs in this zone, close to the squamocolumnar junction.
Inflammation of the uterine cervix (figure 1.7)
The most common pathological condition affecting a woman's cervix is inflammation. This is caused mostly by infection (usually polymicrobial), and less commonly by foreign bodies (retained tampon, etc.), trauma and chemical irritants such as gels and creams. The infectious agents causing inflammation in the cervix include: Trichomonas vaginalis, Candida albicans; overgrowth of anaerobic bacteria such as Gardnerella vaginalis, G. mobilluncus and peptostreptococcus; other bacterial infections such as Haemophilus ducreyi, Neisseria gonorrhoeae, Chlamydia trachomatis, Escherichia coli, streptococci, and staphylococci; and viral infections such as Herpes simplex.
Columnar epithelium is more prone to infection than squamous epithelium. We use the term cervicitis in this manual to denote all cervicovaginal inflammatory conditions. Clinically, cervicitis may be associated with symptoms such as excessive discharge, itching of the vulva and vagina, pain and a burning sensation during sexual intercourse and lower abdominal pain. Clinical signs include excessive, coloured (greyish, greyish-white, curdy-white (in the case of candidial infection), yellow or greenish-yellow), malodorous or non-odorous, frothy or non-frothy secretions, tender, reddish cervix with or without vesicles, ulcerations and/or fibrosis; the columnar epithelium may look flattened; and there may be excoriation marks on the vulva, vulval erythema and oedema, vagina and inner thigh and perineum. Microscopically, cervicitis is characterized by cellular debris and excessive secretions covering the epithelium, swollen and inflamed cells, desquamation of the glycogen-containing superficial and intermediate cells, epithelial denudation, superficial or deep ulceration and congestion of the underlying cervical stroma. Chronic inflammation results in recurrent ulceration and may lead to healing by fibrosis.
A diagnosis of cervicitis can be made based on the clinical features. On visual examination, cervicitis due to non-candidial infection may be characterized by vulval erythema and oedema, excoriation marks in the vulva and vagina and a reddish, tender cervix with malodorous, greenish yellow or greyish-white mucopurulent discharge, with or without ulceration. In the case of gonococcal cervicitis, painful urethral discharge is also observed. Candidial cervicitis is characterized by vulval oedema and erythema, excoriation, and thick, curdy-white, non-odorous discharge. Herpes infection is associated with the presence of vesicles and ulcers in the external genitalia, vagina and the cervix, as well as cervical tenderness. Women with non-candidial cervicitis may be treated with a combination of metronidazole 400 mg plus doxycycline 100 mg orally, two times a day for seven days. Those with candidial cervicitis may be treated with clotrimazole or miconazole 200 mg intravaginally, daily for three days.
Columnar epithelium is more prone to infection than squamous epithelium. We use the term cervicitis in this manual to denote all cervicovaginal inflammatory conditions. Clinically, cervicitis may be associated with symptoms such as excessive discharge, itching of the vulva and vagina, pain and a burning sensation during sexual intercourse and lower abdominal pain. Clinical signs include excessive, coloured (greyish, greyish-white, curdy-white (in the case of candidial infection), yellow or greenish-yellow), malodorous or non-odorous, frothy or non-frothy secretions, tender, reddish cervix with or without vesicles, ulcerations and/or fibrosis; the columnar epithelium may look flattened; and there may be excoriation marks on the vulva, vulval erythema and oedema, vagina and inner thigh and perineum. Microscopically, cervicitis is characterized by cellular debris and excessive secretions covering the epithelium, swollen and inflamed cells, desquamation of the glycogen-containing superficial and intermediate cells, epithelial denudation, superficial or deep ulceration and congestion of the underlying cervical stroma. Chronic inflammation results in recurrent ulceration and may lead to healing by fibrosis.
A diagnosis of cervicitis can be made based on the clinical features. On visual examination, cervicitis due to non-candidial infection may be characterized by vulval erythema and oedema, excoriation marks in the vulva and vagina and a reddish, tender cervix with malodorous, greenish yellow or greyish-white mucopurulent discharge, with or without ulceration. In the case of gonococcal cervicitis, painful urethral discharge is also observed. Candidial cervicitis is characterized by vulval oedema and erythema, excoriation, and thick, curdy-white, non-odorous discharge. Herpes infection is associated with the presence of vesicles and ulcers in the external genitalia, vagina and the cervix, as well as cervical tenderness. Women with non-candidial cervicitis may be treated with a combination of metronidazole 400 mg plus doxycycline 100 mg orally, two times a day for seven days. Those with candidial cervicitis may be treated with clotrimazole or miconazole 200 mg intravaginally, daily for three days.

fig 1.7: A schematic diagram of further maturation of immature squamous metaplasia.
Cervical neoplasia
Invasive cervical cancers are usually preceded by a long phase of preinvasive disease, characterized microscopically as a spectrum of precursor lesions progressing from cellular atypia to various grades of cervical intraepithelial neoplasia (CIN) before progression to invasive carcinoma. Epidemiological studies have identified a number of risk factors that contribute to the development of CIN and cervical cancer. These include infection with certain types of human papillomavirus (HPV), sexual intercourse at an early age, multiple sexual partners, multiparity, long-term oral contraceptive use, tobacco smoking, low socioeconomic status, infection with Chlamydia trachomatis, micronutrient deficiency and a diet deficient in vegetables and fruits. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 are strongly associated with CIN and invasive cancer. Persistent infection with one or more of the above HPV types is considered to be a necessary cause for cervical neoplasia.
Infection with one or more of the oncogenic HPV types may result in the integration of the viral genome into the host cellular genome resulting in the formation of cervical neoplastic cells, the proliferation of which leads to various grades of CIN (synonyms: dysplasia or squamous intraepithelial lesions (SIL)), which may progress to invasive cervical cancer. The correlation between the CIN terminology, used in this manual, and other terminologies is given in Table 1.
Infection with one or more of the oncogenic HPV types may result in the integration of the viral genome into the host cellular genome resulting in the formation of cervical neoplastic cells, the proliferation of which leads to various grades of CIN (synonyms: dysplasia or squamous intraepithelial lesions (SIL)), which may progress to invasive cervical cancer. The correlation between the CIN terminology, used in this manual, and other terminologies is given in Table 1.

table 1: Correlation between CIN, dysplasia and the Bethesda terminology.
Cervical intraepithelial neoplasia
There are no specific symptoms or visible signs associated with CIN. However, the presence of CIN may be suspected by the naked-eye detection of well defined, acetowhite areas in the transformation zone, close to or abutting the squamocolumnar junction, after the application of 3-5% acetic acid or of well defined mustard or saffron yellow iodine non-uptake areas in the transformation zone after application of Lugol's iodine solution.
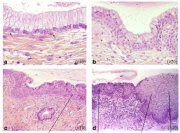
The final diagnosis of CIN is established by histopathological examination of tissue specimens from the cervix. The undifferentiated cells in CIN are characterized by enlarged nuclei, increased intensity of nuclear staining, nuclear polymorphism and variation in nuclear size, and a decreased amount of cytoplasm, resulting in a higher nuclear cytoplasmic ratio. The proportion of the thickness of the epithelium showing undifferentiated cells is used for grading CIN. In CIN 1 the undifferentiated cells are confined to the deeper layers (lower third) of the epithelium (figure 1.8). Mitotic figures are present, but not very numerous. CIN 2 is characterized by dysplastic cellular changes mostly restricted to the lower half or the lower two-thirds of the epithelium, with more marked nuclear abnormalities than in CIN 1 (figure 1.9). Mitotic figures may be seen throughout the lower half of the epithelium. In CIN 3, differentiation and stratification may be totally absent or present only in the superficial quarter of the epithelium with numerous mitotic figures (figure 1.10). Nuclear abnormalities extend throughout the thickness of the epithelium. Many mitotic figures have abnormal forms.
It is well established that most CIN 1 lesions are transient; most of them regress to normal, within relatively short periods, or do not progress to higher grades. High-grade CIN (CIN 2-3), on the other hand, carries a much higher probability of progressing to invasive cancer, although a large proportion of such lesions also regress or persist. It is assumed that the mean interval for progression of cervical precursors to invasive cancer may be as long as 10 to 20 years.
Women with CIN are treated with cryotherapy, loop electrosurgical excision procedure (LEEP) or cold-knife conization. Women with CIN 1 may be advised to undergo immediate treatment (e.g., in situations where follow-up of women cannot be assured) or treated later if two follow-up visits at six or nine months apart reveal persistent or progressive disease.
The precursor lesion that arises from the columnar epithelium is referred to as adenocarcinoma in situ (AIS). In AIS, normal columnar epithelium is replaced by abnormal epithelium showing abnormal, irregularly arranged cells with increased size of cells and nuclei, nuclear hyperchromasia, mitotic activity, reduction of cytoplasmic mucin expression and cellular stratification.
The final diagnosis of CIN is established by histopathological examination of tissue specimens from the cervix. The undifferentiated cells in CIN are characterized by enlarged nuclei, increased intensity of nuclear staining, nuclear polymorphism and variation in nuclear size, and a decreased amount of cytoplasm, resulting in a higher nuclear cytoplasmic ratio. The proportion of the thickness of the epithelium showing undifferentiated cells is used for grading CIN. In CIN 1 the undifferentiated cells are confined to the deeper layers (lower third) of the epithelium (figure 1.8). Mitotic figures are present, but not very numerous. CIN 2 is characterized by dysplastic cellular changes mostly restricted to the lower half or the lower two-thirds of the epithelium, with more marked nuclear abnormalities than in CIN 1 (figure 1.9). Mitotic figures may be seen throughout the lower half of the epithelium. In CIN 3, differentiation and stratification may be totally absent or present only in the superficial quarter of the epithelium with numerous mitotic figures (figure 1.10). Nuclear abnormalities extend throughout the thickness of the epithelium. Many mitotic figures have abnormal forms.
It is well established that most CIN 1 lesions are transient; most of them regress to normal, within relatively short periods, or do not progress to higher grades. High-grade CIN (CIN 2-3), on the other hand, carries a much higher probability of progressing to invasive cancer, although a large proportion of such lesions also regress or persist. It is assumed that the mean interval for progression of cervical precursors to invasive cancer may be as long as 10 to 20 years.
Women with CIN are treated with cryotherapy, loop electrosurgical excision procedure (LEEP) or cold-knife conization. Women with CIN 1 may be advised to undergo immediate treatment (e.g., in situations where follow-up of women cannot be assured) or treated later if two follow-up visits at six or nine months apart reveal persistent or progressive disease.
The precursor lesion that arises from the columnar epithelium is referred to as adenocarcinoma in situ (AIS). In AIS, normal columnar epithelium is replaced by abnormal epithelium showing abnormal, irregularly arranged cells with increased size of cells and nuclei, nuclear hyperchromasia, mitotic activity, reduction of cytoplasmic mucin expression and cellular stratification.

fig 1.8: Histology of CIN 1: The dyplastic cells are confined to the lower third of the epithelium (x20)

fig 1.9: Histology of CIN 2: Atypical cells are found mostly in the lower two-thirds of the epithelium (x10)

fig 1.10: Histology of CIN 3: Dysplastic cells are distributed in the full thickness of the epithelium with loss of polarity of cells (x20)
Invasive cancer
In very early phases of invasion, cervical cancer may not be associated with obvious symptoms and signs, and, therefore, is known as preclinical invasive cancer. Women with moderately advanced or advanced invasive cervical cancer often present with one or more of the following symptoms: intermenstrual bleeding, postcoital bleeding, excessive seropurulent discharge, recurrent cystitis, backache, lower abdominal pain, oedema of the lower extremities, obstructive uropathy, bowel obstruction, breathlessness due to severe anaemia and cachexia.
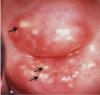
As the stromal invasion progresses, the disease becomes clinically obvious, showing several growth patterns, which are visible on speculum examination. Early lesions may present as a rough, reddish, granular area that bleeds on touch (figure 1.11) More advanced cancers may present as a proliferating, bulging, mushroom- or cauliflower-like growth with bleeding and foul-smelling discharge (figure 1.12). Occasionally they may present without much surface growth, resulting in a grossly enlarged, irregular cervix with a rough, granular surface.
As the invasion continues further, it may involve the vagina, parametrium, pelvic sidewall, bladder and rectum. Compression of the ureter, due to advanced local disease, causes ureteral obstruction which results in hydronephrosis and, ultimately, renal failure. Regional lymph node metastasis occurs along with local invasion. Metastatic cancer in para-aortic nodes may extend through the node capsule and directly invade the vertebrae and nerve roots causing back pain. Direct invasion of the branches of the sciatic nerve roots causes low back pain and leg aches, and encroachment of the pelvic wall veins and lymphatics causes oedema of the lower limbs. Distant metastases occur late in the disease, usually involving para-aortic nodes, lungs, liver, bone and other structures.
Histologically, approximately 90-95% of invasive cervical cancers in developing countries are squamous cell cancers (figure 1.13) and 2 - 8% are adenocarcinomas (figure 1.14). It is obligatory that all invasive cancers be clinically staged. The most widely used staging system for cervical cancer was developed by the International Federation of Gynecology and Obstetrics (FIGO) (see Appendix 1). This is primarily a clinical staging system based on tumour size and extension of the disease in the pelvis. The extent of growth of cancer is assessed clinically, as well as by various investigations, to categorize the disease stages I through IV. Stage I represents growth localized on the cervix, while stage IV represents the growth phase in which the cancer has spread to distant organs by metastasis.
Women with early invasive cancers (stages I, II A) may be treated with radical surgery and/or radiotherapy. Those with stage IIB and III cancers should be treated with radiotherapy with or without cisplatinum-based chemotherapy. Women with stage IV cancers are usually treated with palliative radiotherapy and/or chemotherapy and with symptomatic measures.
As the stromal invasion progresses, the disease becomes clinically obvious, showing several growth patterns, which are visible on speculum examination. Early lesions may present as a rough, reddish, granular area that bleeds on touch (figure 1.11) More advanced cancers may present as a proliferating, bulging, mushroom- or cauliflower-like growth with bleeding and foul-smelling discharge (figure 1.12). Occasionally they may present without much surface growth, resulting in a grossly enlarged, irregular cervix with a rough, granular surface.
As the invasion continues further, it may involve the vagina, parametrium, pelvic sidewall, bladder and rectum. Compression of the ureter, due to advanced local disease, causes ureteral obstruction which results in hydronephrosis and, ultimately, renal failure. Regional lymph node metastasis occurs along with local invasion. Metastatic cancer in para-aortic nodes may extend through the node capsule and directly invade the vertebrae and nerve roots causing back pain. Direct invasion of the branches of the sciatic nerve roots causes low back pain and leg aches, and encroachment of the pelvic wall veins and lymphatics causes oedema of the lower limbs. Distant metastases occur late in the disease, usually involving para-aortic nodes, lungs, liver, bone and other structures.
Histologically, approximately 90-95% of invasive cervical cancers in developing countries are squamous cell cancers (figure 1.13) and 2 - 8% are adenocarcinomas (figure 1.14). It is obligatory that all invasive cancers be clinically staged. The most widely used staging system for cervical cancer was developed by the International Federation of Gynecology and Obstetrics (FIGO) (see Appendix 1). This is primarily a clinical staging system based on tumour size and extension of the disease in the pelvis. The extent of growth of cancer is assessed clinically, as well as by various investigations, to categorize the disease stages I through IV. Stage I represents growth localized on the cervix, while stage IV represents the growth phase in which the cancer has spread to distant organs by metastasis.
Women with early invasive cancers (stages I, II A) may be treated with radical surgery and/or radiotherapy. Those with stage IIB and III cancers should be treated with radiotherapy with or without cisplatinum-based chemotherapy. Women with stage IV cancers are usually treated with palliative radiotherapy and/or chemotherapy and with symptomatic measures.

fig 1.11: Early invasive cervical cancer: note the irregular, granular, nodular surface with bleeding on touch.

fig 1.12: Advanced invasive cervical cancer: note the bulging, cauliflower-like, ulceroproliferative growth with bleeding and necrosis.

fig 1.13: Histology - Keratinizing well differentiated invasive squamous cell carcinoma. Note the stroma is infiltrated by sheets of malignant cells (x10)

fig 1.14: Histology - Well differentiated invasive adenocarcinoma. Note the malignant cells lining the cervical crypts (x20)
Other conditions
Leukoplakia (hyperkeratosis) is a well demarcated white area on the cervix (before the application of acetic acid), due to keratosis, visible to the naked eye. Usually leukoplakia is idiopathic, but it may also be caused by chronic foreign body irritation, HPV infection, or squamous neoplasia. Condylomata or genital warts are often multiple, exophytic lesions that are usually found on the cervix, and occasionally in the vagina and on the vulva, caused by infection with certain HPV types such as 6 and 11. They may also present as a diffuse, greyish-white, lesion involving areas of the cervix and vagina. Condylomata may be obvious to the naked eye (before the application of acetic acid).
Pathophysiological basis of VIA
Application of 5% acetic acid, is believed to cause a reversible coagulation, or precipitation of the cellular proteins. It also causes swelling of the epithelial tissue, columnar and any abnormal squamous epithelial areas in particular and dehydration of the cells and it helps in coagulating and clearing the mucous secretions on the cervix. The normal squamous epithelium appears pink and the columnar epithelium red, due to the reflection of light from the underlying stroma, which is rich in blood vessels. If the epithelium contains a lot of cellular proteins, acetic acid coagulates these proteins, which may obliterate the colour of the stroma. The resulting acetowhitening is seen distinctly as compared with the normal pinkish colour of the surrounding normal squamous epithelium of the cervix, an effect that is commonly visible to the naked eye. Thus, the effect of acetic acid depends upon the amount of cellular proteins present in the epithelium. Areas of increased nuclear activity and DNA content exhibit the most dramatic white colour change.
When acetic acid is applied to normal squamous epithelium, little coagulation occurs in the superficial cell layer, as this is sparsely nucleated. Though the deeper cells contain more nuclear protein, the acetic acid may not penetrate sufficiently and, hence, the resulting precipitation is not sufficient to obliterate the colour of the underlying stroma. Areas of CIN and invasive cancer undergo maximal coagulation due to their higher content of nuclear protein (in view of the large number of undifferentiated cells contained in the epithelium) and prevent light from passing through the epithelium. As a result, the sub-epithelial vessel pattern is obliterated and the epithelium appears densely white. In CIN, acetowhitening is restricted to the transformation zone close to the squamocolumnar junction, while in cancer it often involves the entire cervix.
The acetowhite appearance is not unique to CIN and early cancer. It is also seen in other conditions when increased nuclear protein is present, as in immature squamous metaplasia, in healing and regenerating epithelium (associated with inflammation), leukoplakia (hyperkeratosis) and condyloma. While the acetowhite epithelium associated with CIN and early invasive cancer is more dense, thick and opaque with well demarcated margins from the surrounding normal epithelium, the acetowhitening associated with immature squamous metaplasia, inflammation and regenerating epithelium is less pale, thin, often translucent, and patchy with ill-defined margins. Acetowhitening due to inflammation and healing is usually distributed widely in the cervix, not restricted to the transformation zone and may quickly disappear (within a minute). Leukoplakia and condylomata appear intensely greyish-white after the application of acetic acid.
The acetic acid effect reverses much more slowly in CIN lesions and in early pre-clinical invasive cancer than in immature squamous metaplasia and inflammation. It appears rapidly and may last for 3-5 minutes in the case of CIN 2-3 and invasive cancer.
When acetic acid is applied to normal squamous epithelium, little coagulation occurs in the superficial cell layer, as this is sparsely nucleated. Though the deeper cells contain more nuclear protein, the acetic acid may not penetrate sufficiently and, hence, the resulting precipitation is not sufficient to obliterate the colour of the underlying stroma. Areas of CIN and invasive cancer undergo maximal coagulation due to their higher content of nuclear protein (in view of the large number of undifferentiated cells contained in the epithelium) and prevent light from passing through the epithelium. As a result, the sub-epithelial vessel pattern is obliterated and the epithelium appears densely white. In CIN, acetowhitening is restricted to the transformation zone close to the squamocolumnar junction, while in cancer it often involves the entire cervix.
The acetowhite appearance is not unique to CIN and early cancer. It is also seen in other conditions when increased nuclear protein is present, as in immature squamous metaplasia, in healing and regenerating epithelium (associated with inflammation), leukoplakia (hyperkeratosis) and condyloma. While the acetowhite epithelium associated with CIN and early invasive cancer is more dense, thick and opaque with well demarcated margins from the surrounding normal epithelium, the acetowhitening associated with immature squamous metaplasia, inflammation and regenerating epithelium is less pale, thin, often translucent, and patchy with ill-defined margins. Acetowhitening due to inflammation and healing is usually distributed widely in the cervix, not restricted to the transformation zone and may quickly disappear (within a minute). Leukoplakia and condylomata appear intensely greyish-white after the application of acetic acid.
The acetic acid effect reverses much more slowly in CIN lesions and in early pre-clinical invasive cancer than in immature squamous metaplasia and inflammation. It appears rapidly and may last for 3-5 minutes in the case of CIN 2-3 and invasive cancer.
Pathophysiological basis of VILI
Squamous metaplastic epithelium is glycogenated, whereas CIN and invasive cancer cells contain little or no glycogen. Columnar epithelium does not contain glycogen. Immature squamous metaplastic epithelium usually lacks glycogen or, occasionally, may be partially glycogenated. Iodine is glycophilic and hence the application of iodine solution results in uptake of iodine in glycogen-containing epithelium. Therefore, the normal glycogen-containing squamous epithelium stains mahogany brown or black after application of iodine. Columnar epithelium does not take up iodine and remains unstained, but may look slightly discoloured due to a thin film of iodine solution; areas of immature squamous metaplastic epithelium may remain unstained with iodine or may be only partially stained. If there is shedding (or erosion) of superficial and intermediate cell layers associated with inflammatory conditions of the squamous epithelium, these areas do not stain with iodine and remain distinctly colourless in a surrounding black or brown background. Areas of CIN and invasive cancer do not take up iodine (as they lack glycogen) and appear as thick mustard-yellow or saffron-coloured areas. Areas with leukoplakia (hyperkeratosis) do not stain with iodine either, and condylomata may not, or occasionally may only partially, stain with iodine.