Home / Training / Manuals / Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual / Chapter 12: Treatment of cervical intraepithelial neoplasia by cryotherapy
 figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment...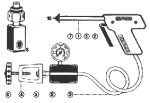 figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 13.3: Instrument tray for L...
figure 13.3: Instrument tray for L... figure 4.9: Vaginal speculum cover...
figure 4.9: Vaginal speculum cover...
 figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment... figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 12.4: Cryotherapy unit conn...
figure 12.4: Cryotherapy unit conn...
 table 12.1: Eligibility criteria f...
table 12.1: Eligibility criteria f...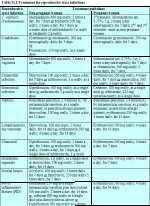 table 11.1: Treatment for reproduc...
table 11.1: Treatment for reproduc...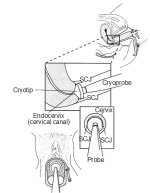 figure 12.5: Positioning of the cr...
figure 12.5: Positioning of the cr... figure 12.6: Cryofreezing in progr...
figure 12.6: Cryofreezing in progr...
 figure 12.5: Positioning of the cr...
figure 12.5: Positioning of the cr... figure 12.6: Cryofreezing in progr...
figure 12.6: Cryofreezing in progr... figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment... figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 12.4: Cryotherapy unit conn...
figure 12.4: Cryotherapy unit conn...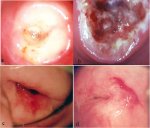 figure 12.7: (a) The iceball on th...
figure 12.7: (a) The iceball on th...
 figure 12.7: (a) The iceball on th...
figure 12.7: (a) The iceball on th...
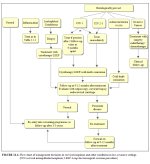 figure 11.1: Flow chart of managem...
figure 11.1: Flow chart of managem...
Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual, Edited by J.W. Sellors and R. Sankaranarayanan
Chapter 12: Treatment of cervical intraepithelial neoplasia by cryotherapy
Filter by language: English / Français / Español / Portugues / 中文- Cryotherapy and loop electrosurgical excision procedure (LEEP) are suitable and effective treatment options for CIN in both low- and high-resource settings, as both require less financial investment for equipment and maintenance, and both can be learnt in a short period of time.
- Compared to equipment required for LEEP, cryotherapy equipment costs substantially less.
- Cryotherapy relies on a steady supply of compressed refrigerant gases (N2O or CO2) in transportable cylinders. Cryotherapy is not adequate to treat lesions involving the endocervix.
- If excellent contact between the cryoprobe tip and the ectocervix is achieved, N2O-based cryotherapy will achieve –89°C and CO2-based system will achieve –68°C at the core of the ice ball and temperatures around –20°C at the edges. Cells reduced to –20°C for one or more minutes will undergo cryonecrosis.
- Healing takes place throughout the first six weeks after cryotherapy. Women may experience watery vaginal discharge for 3-4 weeks after treatment.
- Women should be advised not to use a vaginal douche, tampons or have sexual intercourse for one month after treatment.
- Cryotherapy may increase the transmissibility of HIV infection and using condoms is an effective means of prevention.
- Treatment failure is observed in about 5-10% of women.
Ablative and excisional treatments constitute two forms of out-patient surgical treatment of cervical intraepithelial neoplasia (CIN). Cryotherapy, electrocoagulation, cold coagulation and laser ablation are different methods of ablative treatment of CIN. The loop electrosurgical excision procedure (LEEP), using thin wire loop electrodes and long needle electrode electro-surgical cylindrical excision are the major forms of out-patient excisional treatment of CIN.
Of all available and effective treatments of CIN, cryotherapy and LEEP are appropriate for both high- and low-resource settings for several reasons and, hence, only these two methods are discussed in this field manual. First, they require the least financial investment for equipment, maintenance and repair. Second, once colposcopy has been mastered, cryotherapy and LEEP can be quickly learned and result in high cure rates and few complications. Other surgical techniques that are based on the laser or electrocoagulation are beyond the scope of this manual and the learner is referred to excellent books that have been written on their use ( Wright et al., 1992; Wright et al., 19.5; Singer & Monaghan, 2000).
The primary concern in treating CIN by ablative (destructive) or excisional techniques is whether the treatment will be adequate to eradicate any CIN that has extended down into the crypts underlying the neoplastic epithelium. The possible depth of crypt involvement increases with the severity of the CIN. A treatment that is effective to a depth of 7 mm is necessary to destroy CIN 3. The total linear extent of the lesion is also a factor to be considered. The linear extent of a lesion is the sum of its two distances, each measured from a reference point at the external os: the distance to the proximal edge (towards or into the canal) and the distance to the distal edge of the lesion (away from the canal). The average linear extent is 7.5 mm (range 2 to 22 mm) with 85 to 90% of lesions entirely visible externally on the transformation zone ( Wright et al., 19.5). Vaginal extension is present in no more than 5% of patients.
The principles and practice of cryotherapy are discussed in this chapter and LEEP is described in the next chapter. Cryotherapy equipment (Figures 12.1, 12.2, 12.3.jpg&leg=')">and 12.3) costs substantially less to buy and to maintain than that required for LEEP. Cryotherapy does not require a source of electricity as LEEP does, but relies instead on a supply of easily transportable tanks of highly compressed refrigerant gas. After the vaginal speculum is in place and the cervix has been visualized, both procedures take approximately 15 minutes from start to finish. Ancillary equipment is required for LEEP, but not for cryotherapy for several reasons. Although the performance of cryotherapy usually does not require a local anaesthetic, LEEP does require several injections of a local anaesthetic into the ectocervix. LEEP generates smoke that remains in the vagina unless it is evacuated by a vacuum system to allow an unobstructed view of the operative field. The third type of ancillary equipment required for LEEP is an electrically insulated vaginal speculum (and insulated vaginal side-wall retractor, if necessary) (Figure 13.3) or a metallic speculum insulated with latex condom (Figure 4.9) to prevent an electrical injury (shock or thermal injury) to the patient or the operator if the loop or the ball electrode accidentally touches the instrument. Since a metallic vaginal speculum conducts electricity, it may lead to an electrical injury to the vagina if the loop accidentally comes into contact with these metallic instruments. Insulated vaginal specula and insulated vaginal side-wall retractors are more expensive than non-insulated ones.
In contrast to LEEP, which is an excisional technique, cryotherapy is an ablative one. In practical terms, this means that there will be no pathology specimen to evaluate after cryotherapy which obviously has an immediate cost saving. Proponents of LEEP appreciate the feedback of information if there is a pathological examination of the LEEP excised tissue. This feedback allows a reassessment of not only the most severe grade of lesion present, but also the adequacy of excision (whether excisional margins are involved).
The main limitation of cryotherapy is that it is not adequate to treat lesions that are not wholly located on the ectocervix, yet involve the endocervical canal. In contrast, LEEP can adequately excise the majority of cervical lesions, whether or not the canal is involved. Meta-analysis of randomized clinical trials that evaluated the comparative effectiveness of cryotherapy with therapies such as LEEP, conization and laser, have concluded that the above treatments are equally effective in controlling CIN ( Nuovo et al., 2000; Martin-Hirch et al., 2000). From the foregoing comparisons and contrasts, it is empirically clear that the most practical and cost-effective method of treatment of CIN in low-resource settings is cryotherapy, provided the lesion is wholly ectocervical in location. LEEP is the treatment of choice if the lesion involves the endocervical canal (see Chapter 13).
Since LEEP is technically more demanding than cryotherapy, we suggest that colposcopists should first demonstrate competence with cryotherapy before they perform LEEP.
If living tissue is frozen to a temperature of -20°C or lower for at least 1 minute, cryonecrosis ensues. Several features distinguish this process: intra- and extra-cellular crystallization, dehydration, thermal shock, vascular stasis and protein denaturation. A rapid freeze followed by a slow thaw is the most damaging to cells, especially neoplastic cells. A sequence of two freeze-thaw cycles (freeze-thaw-freeze-thaw) may produce more tissue destruction than a single cycle.
The cryotherapy technique uses a cryoprobe with a tip made of highly conductive metal (usually silver and copper), that makes direct surface contact with the ectocervical lesion. A substantial drop in temperature is achieved when a compressed refrigerant gas is allowed to expand through a small aperture in the cryoprobe. Nitrous oxide (N2O) or carbon dioxide (CO2) are the refrigerants of choice, as both provide excellent thermal transfer when circulating in the probe tip.
Of all available and effective treatments of CIN, cryotherapy and LEEP are appropriate for both high- and low-resource settings for several reasons and, hence, only these two methods are discussed in this field manual. First, they require the least financial investment for equipment, maintenance and repair. Second, once colposcopy has been mastered, cryotherapy and LEEP can be quickly learned and result in high cure rates and few complications. Other surgical techniques that are based on the laser or electrocoagulation are beyond the scope of this manual and the learner is referred to excellent books that have been written on their use ( Wright et al., 1992; Wright et al., 19.5; Singer & Monaghan, 2000).
The primary concern in treating CIN by ablative (destructive) or excisional techniques is whether the treatment will be adequate to eradicate any CIN that has extended down into the crypts underlying the neoplastic epithelium. The possible depth of crypt involvement increases with the severity of the CIN. A treatment that is effective to a depth of 7 mm is necessary to destroy CIN 3. The total linear extent of the lesion is also a factor to be considered. The linear extent of a lesion is the sum of its two distances, each measured from a reference point at the external os: the distance to the proximal edge (towards or into the canal) and the distance to the distal edge of the lesion (away from the canal). The average linear extent is 7.5 mm (range 2 to 22 mm) with 85 to 90% of lesions entirely visible externally on the transformation zone ( Wright et al., 19.5). Vaginal extension is present in no more than 5% of patients.
The principles and practice of cryotherapy are discussed in this chapter and LEEP is described in the next chapter. Cryotherapy equipment (Figures 12.1, 12.2, 12.3.jpg&leg=')">and 12.3) costs substantially less to buy and to maintain than that required for LEEP. Cryotherapy does not require a source of electricity as LEEP does, but relies instead on a supply of easily transportable tanks of highly compressed refrigerant gas. After the vaginal speculum is in place and the cervix has been visualized, both procedures take approximately 15 minutes from start to finish. Ancillary equipment is required for LEEP, but not for cryotherapy for several reasons. Although the performance of cryotherapy usually does not require a local anaesthetic, LEEP does require several injections of a local anaesthetic into the ectocervix. LEEP generates smoke that remains in the vagina unless it is evacuated by a vacuum system to allow an unobstructed view of the operative field. The third type of ancillary equipment required for LEEP is an electrically insulated vaginal speculum (and insulated vaginal side-wall retractor, if necessary) (Figure 13.3) or a metallic speculum insulated with latex condom (Figure 4.9) to prevent an electrical injury (shock or thermal injury) to the patient or the operator if the loop or the ball electrode accidentally touches the instrument. Since a metallic vaginal speculum conducts electricity, it may lead to an electrical injury to the vagina if the loop accidentally comes into contact with these metallic instruments. Insulated vaginal specula and insulated vaginal side-wall retractors are more expensive than non-insulated ones.
In contrast to LEEP, which is an excisional technique, cryotherapy is an ablative one. In practical terms, this means that there will be no pathology specimen to evaluate after cryotherapy which obviously has an immediate cost saving. Proponents of LEEP appreciate the feedback of information if there is a pathological examination of the LEEP excised tissue. This feedback allows a reassessment of not only the most severe grade of lesion present, but also the adequacy of excision (whether excisional margins are involved).
The main limitation of cryotherapy is that it is not adequate to treat lesions that are not wholly located on the ectocervix, yet involve the endocervical canal. In contrast, LEEP can adequately excise the majority of cervical lesions, whether or not the canal is involved. Meta-analysis of randomized clinical trials that evaluated the comparative effectiveness of cryotherapy with therapies such as LEEP, conization and laser, have concluded that the above treatments are equally effective in controlling CIN ( Nuovo et al., 2000; Martin-Hirch et al., 2000). From the foregoing comparisons and contrasts, it is empirically clear that the most practical and cost-effective method of treatment of CIN in low-resource settings is cryotherapy, provided the lesion is wholly ectocervical in location. LEEP is the treatment of choice if the lesion involves the endocervical canal (see Chapter 13).
Since LEEP is technically more demanding than cryotherapy, we suggest that colposcopists should first demonstrate competence with cryotherapy before they perform LEEP.
If living tissue is frozen to a temperature of -20°C or lower for at least 1 minute, cryonecrosis ensues. Several features distinguish this process: intra- and extra-cellular crystallization, dehydration, thermal shock, vascular stasis and protein denaturation. A rapid freeze followed by a slow thaw is the most damaging to cells, especially neoplastic cells. A sequence of two freeze-thaw cycles (freeze-thaw-freeze-thaw) may produce more tissue destruction than a single cycle.
The cryotherapy technique uses a cryoprobe with a tip made of highly conductive metal (usually silver and copper), that makes direct surface contact with the ectocervical lesion. A substantial drop in temperature is achieved when a compressed refrigerant gas is allowed to expand through a small aperture in the cryoprobe. Nitrous oxide (N2O) or carbon dioxide (CO2) are the refrigerants of choice, as both provide excellent thermal transfer when circulating in the probe tip.
 figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment... figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 13.3: Instrument tray for L...
figure 13.3: Instrument tray for L... figure 4.9: Vaginal speculum cover...
figure 4.9: Vaginal speculum cover...Cryotherapy equipment (Figures 12.1, 12.2, 12.3 and 12.4)
The cryotherapy unit consists of a compressed gas cylinder (tank), a yoke with a tightening knob and an inlet of gas to connect the gas cylinder to the cryotherapy gun through a flexible gas-conveying tube, a pressure gauge showing the cylinder gas pressure, an outlet silencer, a cryotherapy gun with handle grip, a gas trigger to allow the gas to be released to the cryotherapy probe at high pressure and the cryotherapy probe. In most equipment, the pressure gauge shows three colour zones: yellow, green and red. When the gas cylinder is opened, if the pressure indicator in the gauge moves to the green zone, there is adequate gas pressure for treatment; if the needle remains in the yellow zone, the pressure is too low and the gas cylinder should be changed before commencing treatment; if the needle moves to the red zone, excess pressure is indicated and this excess pressure should be released. One should consult the manual provided by the manufacturer thoroughly for operational instructions.
 figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment... figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 12.4: Cryotherapy unit conn...
figure 12.4: Cryotherapy unit conn...Cryotherapy for ectocervical lesions
It is advisable to use the largest cylinder of refrigerant gas possible, so that a sufficient amount of refrigerant is available to complete the treatment and the pressure forcing the refrigerant through the probe tip is maintained at a high level so that the effectiveness of the procedure is maintained. Standard-size tanks only allow adequate pressure to treat three women. A large tank has the advantage of treating more women, but transport from clinic to clinic may pose a problem.
If excellent contact is achieved between the probe tip and the ectocervix (Figures 12.5 and 12.6b), a nitrous oxide-based cryotherapy will achieve temperatures of about -89°C and the carbon dioxide- based system will achieve –68oC at the core of the tissue ice ball. The temperature at the edges of the frozen tissue may be around –20oC. Cells held at –20oC for one minute or more will undergo cryonecrosis. The minimum temperature at the probe tip for effective freezing should be –60oC. It is critical to establish and maintain good contact throughout the procedure between the probe tip and the tissue - poor contact means a relatively large variation in the temperatures achieved within the ice ball and therefore variable effectiveness in the target tissue.
If excellent contact is achieved between the probe tip and the ectocervix (Figures 12.5 and 12.6b), a nitrous oxide-based cryotherapy will achieve temperatures of about -89°C and the carbon dioxide- based system will achieve –68oC at the core of the tissue ice ball. The temperature at the edges of the frozen tissue may be around –20oC. Cells held at –20oC for one minute or more will undergo cryonecrosis. The minimum temperature at the probe tip for effective freezing should be –60oC. It is critical to establish and maintain good contact throughout the procedure between the probe tip and the tissue - poor contact means a relatively large variation in the temperatures achieved within the ice ball and therefore variable effectiveness in the target tissue.
 table 12.1: Eligibility criteria f...
table 12.1: Eligibility criteria f... table 11.1: Treatment for reproduc...
table 11.1: Treatment for reproduc... figure 12.5: Positioning of the cr...
figure 12.5: Positioning of the cr... figure 12.6: Cryofreezing in progr...
figure 12.6: Cryofreezing in progr...The step-by-step approach to cryotherapy (Figures 12.5 and 12.6)
A woman should meet the eligibility criteria in Table 12.1. Generally, it is preferable to have the diagnosis of CIN firmly established before cryotherapy is performed. However, there may be exceptions to this general rule. For example, in developing countries, women may be offered treatment at their first colposcopy visit to maximize treatment coverage (otherwise patients lost to follow-up would not receive treatment for lesions) on the basis of a colposcopy diagnosis. However, directed biopsy may be carried out before instituting cryotherapy, so that a histological diagnosis will be available to establish the nature of the lesion treated a posteriori. The consequences of such an approach in terms of possible over-treatment or unnecessary treatment, as well as the side-effects and complications of the treatment procedure, should be explained and informed consent obtained.
The provider should be familiar with the cryotherapy equipment and its different components (Figures 12.1, 12.2, 12.3 and 12.4) that will be used in a given setting. The instructions for operational use and safety provided by the manufacturer should be read carefully. The safety regulations should be strictly followed. Before cryotherapy is initiated, the gas tank pressure should be checked to ensure that it is sufficient to provide an effective flow of the refrigerant through the probe tip for the required duration of treatment. One should follow the instruction of the manufacturer in this regard. In most models of cryotherapy equipment, a green zone in the pressure gauge indicates adequate pressure (40-70 kg per cm2) and a yellow zone indicates low pressure (less than 40 kg/cm2). If there is adequate gas pressure in the cylinder, the indicator moves to the green zone in the gauge, after the cylinder is opened to release the gas. If the pressure is low, there will be insufficient freezing to give the required extent of cryonecrosis. The minimum working pressure shown on the gauge should be 40 kg/cm2, and the freezing will be inadequate if the pressure falls below this level. In such an event, the gas cylinder should be changed before continuing treatment.
If the woman is returning to the clinic on a second visit (after histological confirmation) for treatment, colposcopic assessment should be done immediately before cryotherapy to confirm that the location and linear extent of the lesion are amenable to effective cryotherapy.
The physician or the nurse should explain the treatment procedure to the woman and reassure her. This is important to help the woman to relax during the procedure. After ensuring she has emptied her bladder, she should be placed in a modified lithotomy position and the cervix should be exposed with the largest speculum that can be introduced comfortably. The secretions are removed with a cotton swab soaked in saline. Then 5% acetic acid is applied and the cervix is examined with the colposcope. Following this, Lugol’s iodine is applied to delineate the limits of the lesion. There is no need for local anaesthesia when performing cryotherapy.
The cryoprobe surface is wiped with saline to ensure adequate thermal contact with the cervix and optimal lowering of the tissue temperature. The cryotherapy probe tip is then firmly applied, with the centre of the tip on the os. It is obligatory to ensure that the vaginal walls are not in contact with the cryoprobe tip. The timer is then set and the gas trigger in the cryogun is released or squeezed to cool the cryoprobe in contact with the cervix. The gas escapes through the pressure gauge with a hissing noise. One should be able to observe ice being formed on the tip of the cryoprobe and on the cervix as freezing progresses. Make sure that the probe adequately covers the lesion and the tip does not inadvertently contact and freeze any part of the vagina during the procedure.
Cryotherapy should consist of two sequential freeze-thaw cycles, each cycle consisting of 3 minutes of freezing followed by 5 minutes of thawing (3 minutes freeze-5 minutes thaw-3 minutes freeze-thaw). The treatment time should be monitored using a stop watch. Adequate freezing has been achieved when the margin of the ice ball extends 4-5 mm past the outer edge of the cryotip. This will ensure that cryonecrosis occurs down to at least 5 mm depth. To achieve this effect evenly throughout the treatment field, it is extremely important to establish and maintain excellent contact between the probe tip and the ectocervical surface. Once the second freeze for 3 minutes is completed, allow time for adequate thawing before removing the probe from the cervix. When thawing is completed, the ice formation on the cryoprobe tip is totally cleared and the probe is removed by gently rotating on the cervix. Do not attempt to remove the probe tip from the cervix until complete thawing has occurred. After removing the probe, examine the cervix for any bleeding. The appearance of the cervix immediately after cryotherapy is shown in Figure 12.7a. Note the iceball formed in the cervix. The vagina should not be packed with gauze or cotton after cryotherapy to allow the secretions to escape. Women may be provided with a supply of sanitary pads to prevent the secretions staining their clothes.
After use, the probe tip should be wiped with 60-90% ethyl or isopropyl alcohol and then cleaned well with boiled water and disinfected with 2% glutaradehyde (see Chapter 14) and kept dry. After the procedure is completed, the cryogun, tubing, pressure gauge and gas tank should be de-contaminated by wiping with cotton soaked with 60-90% ethyl or isopropyl alcohol.
The provider should be familiar with the cryotherapy equipment and its different components (Figures 12.1, 12.2, 12.3 and 12.4) that will be used in a given setting. The instructions for operational use and safety provided by the manufacturer should be read carefully. The safety regulations should be strictly followed. Before cryotherapy is initiated, the gas tank pressure should be checked to ensure that it is sufficient to provide an effective flow of the refrigerant through the probe tip for the required duration of treatment. One should follow the instruction of the manufacturer in this regard. In most models of cryotherapy equipment, a green zone in the pressure gauge indicates adequate pressure (40-70 kg per cm2) and a yellow zone indicates low pressure (less than 40 kg/cm2). If there is adequate gas pressure in the cylinder, the indicator moves to the green zone in the gauge, after the cylinder is opened to release the gas. If the pressure is low, there will be insufficient freezing to give the required extent of cryonecrosis. The minimum working pressure shown on the gauge should be 40 kg/cm2, and the freezing will be inadequate if the pressure falls below this level. In such an event, the gas cylinder should be changed before continuing treatment.
If the woman is returning to the clinic on a second visit (after histological confirmation) for treatment, colposcopic assessment should be done immediately before cryotherapy to confirm that the location and linear extent of the lesion are amenable to effective cryotherapy.
The physician or the nurse should explain the treatment procedure to the woman and reassure her. This is important to help the woman to relax during the procedure. After ensuring she has emptied her bladder, she should be placed in a modified lithotomy position and the cervix should be exposed with the largest speculum that can be introduced comfortably. The secretions are removed with a cotton swab soaked in saline. Then 5% acetic acid is applied and the cervix is examined with the colposcope. Following this, Lugol’s iodine is applied to delineate the limits of the lesion. There is no need for local anaesthesia when performing cryotherapy.
The cryoprobe surface is wiped with saline to ensure adequate thermal contact with the cervix and optimal lowering of the tissue temperature. The cryotherapy probe tip is then firmly applied, with the centre of the tip on the os. It is obligatory to ensure that the vaginal walls are not in contact with the cryoprobe tip. The timer is then set and the gas trigger in the cryogun is released or squeezed to cool the cryoprobe in contact with the cervix. The gas escapes through the pressure gauge with a hissing noise. One should be able to observe ice being formed on the tip of the cryoprobe and on the cervix as freezing progresses. Make sure that the probe adequately covers the lesion and the tip does not inadvertently contact and freeze any part of the vagina during the procedure.
Cryotherapy should consist of two sequential freeze-thaw cycles, each cycle consisting of 3 minutes of freezing followed by 5 minutes of thawing (3 minutes freeze-5 minutes thaw-3 minutes freeze-thaw). The treatment time should be monitored using a stop watch. Adequate freezing has been achieved when the margin of the ice ball extends 4-5 mm past the outer edge of the cryotip. This will ensure that cryonecrosis occurs down to at least 5 mm depth. To achieve this effect evenly throughout the treatment field, it is extremely important to establish and maintain excellent contact between the probe tip and the ectocervical surface. Once the second freeze for 3 minutes is completed, allow time for adequate thawing before removing the probe from the cervix. When thawing is completed, the ice formation on the cryoprobe tip is totally cleared and the probe is removed by gently rotating on the cervix. Do not attempt to remove the probe tip from the cervix until complete thawing has occurred. After removing the probe, examine the cervix for any bleeding. The appearance of the cervix immediately after cryotherapy is shown in Figure 12.7a. Note the iceball formed in the cervix. The vagina should not be packed with gauze or cotton after cryotherapy to allow the secretions to escape. Women may be provided with a supply of sanitary pads to prevent the secretions staining their clothes.
After use, the probe tip should be wiped with 60-90% ethyl or isopropyl alcohol and then cleaned well with boiled water and disinfected with 2% glutaradehyde (see Chapter 14) and kept dry. After the procedure is completed, the cryogun, tubing, pressure gauge and gas tank should be de-contaminated by wiping with cotton soaked with 60-90% ethyl or isopropyl alcohol.
 figure 12.5: Positioning of the cr...
figure 12.5: Positioning of the cr... figure 12.6: Cryofreezing in progr...
figure 12.6: Cryofreezing in progr... figure 12.1: Cryoprobes, the cryog...
figure 12.1: Cryoprobes, the cryog... figure 12.2: Cryotherapy equipment...
figure 12.2: Cryotherapy equipment... figure 12.3: Components of cryothe...
figure 12.3: Components of cryothe... figure 12.4: Cryotherapy unit conn...
figure 12.4: Cryotherapy unit conn... figure 12.7: (a) The iceball on th...
figure 12.7: (a) The iceball on th...Follow-up after cryotherapy
Women should receive instructions on self-care and what symptoms to expect after treatment. They should be informed that they may experience some mild cramps and a clear or lightly blood-stained watery discharge for up to 4-6 weeks after treatment. Women should be advised not to use a vaginal douche or tampons or to have sexual intercourse for one month after treatment. They should be instructed to report if they have any one of the following symptoms in the six weeks after treatment: fever for more than two days, severe lower abdominal pain, foul-smelling- pus coloured discharge, bleeding with clots or bleeding for over two days. It is preferable to give written instructions on the above aspects and on follow-up.
Healing takes place during the first six weeks after cryotherapy. Granulation tissue is present in the wound during the first 2-3 weeks after cryotherapy (Figure 12.7b), which is followed by re-epithelialization of the surface. Normally, the wound is totally healed within 6-8 weeks of treatment. The appearance of the cervix 3 months and 12 months after cryotherapy is shown in figures 12.7c and 12.7d.
The effect of cryotherapy on the potential transmissibility of human immunodeficiency virus (HIV) infection (to or from women) during the healing phase is not known. HIV-1 shedding in the vaginal secretions after treatment of CIN in HIV-positive women has been demonstrated ( Wright et al., 2001). Therefore, the authors suggest advising all women that cryotherapy may increase the transmissibility of HIV and that using condoms is an effective means of prevention. Condoms should be used for a period of at least four but preferably six weeks. Ideally, a supply of condoms should be available free of charge at colposcopy clinics in settings where HIV infection is endemic.
Appointments should be made for a follow-up visit 9-12 months after treatment. During the follow-up, cytology and/or VIA should be performed, followed by colposcopy and directed biopsy depending upon the colposcopy findings, to assess the regression or persistence of lesions. Retreatment is carried out if lesions persist. Women who are negative for neoplasia may be referred back to a screening programme (if one exists) or advised to undergo follow-up after three or five years.
Healing takes place during the first six weeks after cryotherapy. Granulation tissue is present in the wound during the first 2-3 weeks after cryotherapy (Figure 12.7b), which is followed by re-epithelialization of the surface. Normally, the wound is totally healed within 6-8 weeks of treatment. The appearance of the cervix 3 months and 12 months after cryotherapy is shown in figures 12.7c and 12.7d.
The effect of cryotherapy on the potential transmissibility of human immunodeficiency virus (HIV) infection (to or from women) during the healing phase is not known. HIV-1 shedding in the vaginal secretions after treatment of CIN in HIV-positive women has been demonstrated ( Wright et al., 2001). Therefore, the authors suggest advising all women that cryotherapy may increase the transmissibility of HIV and that using condoms is an effective means of prevention. Condoms should be used for a period of at least four but preferably six weeks. Ideally, a supply of condoms should be available free of charge at colposcopy clinics in settings where HIV infection is endemic.
Appointments should be made for a follow-up visit 9-12 months after treatment. During the follow-up, cytology and/or VIA should be performed, followed by colposcopy and directed biopsy depending upon the colposcopy findings, to assess the regression or persistence of lesions. Retreatment is carried out if lesions persist. Women who are negative for neoplasia may be referred back to a screening programme (if one exists) or advised to undergo follow-up after three or five years.
 figure 12.7: (a) The iceball on th...
figure 12.7: (a) The iceball on th...Management of women for whom cryotherapy fails
Treatment failure is detected in about 5-10% of women during the follow-up in the first year. These persistent, local or multifocal lesions are more likely to occur if the original lesion was large. To rule out the presence of unsuspected invasive carcinoma, it is advisable to biopsy all persistent lesions and then re-treat with cryotherapy, LEEP, or cold- knife conization, as appropriate. Follow-up evaluation may be carried out after 9-12 months in which screening examinations such as cytology and/or VIA and colposcopy should be carried out. Those negative for neoplasia may be referred back to a screening programme (if one exists in the region) or advised to undergo follow-up after three or five years. A management approach for low resource settings is shown in Figure 11.1.
 figure 11.1: Flow chart of managem...
figure 11.1: Flow chart of managem...Adverse effects, complications, and long-term sequelae
Cryotherapy is usually a painless procedure, if women have been properly reassured, their co-operation is obtained, and the procedure is carried out properly. Some women may experience some lower abdominal pain or cramps during and after cryotherapy. Once in a while, a woman may faint due to a vasovagal reaction. In such a situation, there is no need for panic and the women may be revived easily. Bleeding is extremely rare after cryotherapy.
Treated women experience a watery vaginal discharge for about 3-4 weeks after treatment. Vaginal bleeding is extremely unusual; it may be more likely to occur if freezing has been too aggressive and the ice ball has extended well past 5 mm in depth. The risk of post-operative infection is very slight and can probably be reduced further by delaying cryotherapy until any woman with a likely diagnosis of pelvic inflammatory disease (PID), sexually transmitted cervicitis (e.g., chlamydia or gonorrhea), vaginal trichomoniasis or bacterial vaginosis has been adequately treated and recovered. If a woman presents post-operatively with a malodorous discharge, pelvic pain and fever, the discharge may be cultured if possible, and empirical treatment should be prescribed with antibiotics that are effective for PID. Sexual partners should also be treated if the woman is diagnosed with PID, sexually transmitted cervicitis, or trichomoniasis. In developing countries, one may consider providing presumptive treatment with antibiotics routinely after cryotherapy (doxycycline 100 mg orally, two times a day, for seven days and metronidazole 400 mg orally, three times a day, for seven days).
Cervical stenosis occurs in less than 1% of women; reduced mucus production occurs in 5-10% of women. Cryotherapy has no known adverse effect on fertility and pregnancy. Invasive cancer has rarely been reported after cryotherapy, it is usually due to missed diagnosis as a result of poor diagnostic workup before cryotherapy.
Treated women experience a watery vaginal discharge for about 3-4 weeks after treatment. Vaginal bleeding is extremely unusual; it may be more likely to occur if freezing has been too aggressive and the ice ball has extended well past 5 mm in depth. The risk of post-operative infection is very slight and can probably be reduced further by delaying cryotherapy until any woman with a likely diagnosis of pelvic inflammatory disease (PID), sexually transmitted cervicitis (e.g., chlamydia or gonorrhea), vaginal trichomoniasis or bacterial vaginosis has been adequately treated and recovered. If a woman presents post-operatively with a malodorous discharge, pelvic pain and fever, the discharge may be cultured if possible, and empirical treatment should be prescribed with antibiotics that are effective for PID. Sexual partners should also be treated if the woman is diagnosed with PID, sexually transmitted cervicitis, or trichomoniasis. In developing countries, one may consider providing presumptive treatment with antibiotics routinely after cryotherapy (doxycycline 100 mg orally, two times a day, for seven days and metronidazole 400 mg orally, three times a day, for seven days).
Cervical stenosis occurs in less than 1% of women; reduced mucus production occurs in 5-10% of women. Cryotherapy has no known adverse effect on fertility and pregnancy. Invasive cancer has rarely been reported after cryotherapy, it is usually due to missed diagnosis as a result of poor diagnostic workup before cryotherapy.



