Home / Training / Manuals / Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual / Chapter 1: An introduction to the anatomy of the uterine cervix
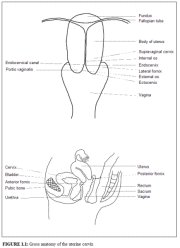 figure 1.1: Gross anatomy of the u...
figure 1.1: Gross anatomy of the u...
 figure 1.2: Stratified squamous ep...
figure 1.2: Stratified squamous ep...
 figure 1.3: Columnar epithelium (x...
figure 1.3: Columnar epithelium (x...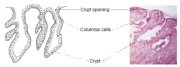 figure 1.4: Crypts of columnar epi...
figure 1.4: Crypts of columnar epi...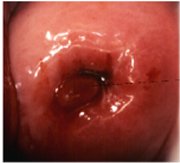 figure 1.5: Cervical polyp
figure 1.5: Cervical polyp
...
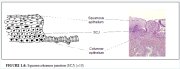 figure 1.6: Squamocolumnar junctio...
figure 1.6: Squamocolumnar junctio...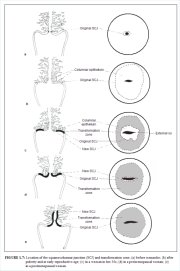 figure 1.7: Location of the squamo...
figure 1.7: Location of the squamo...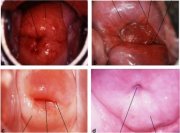 figure 1.8: Location of squamocolu...
figure 1.8: Location of squamocolu...
 figure1.7: Location of the squamoc...
figure1.7: Location of the squamoc... figure1.8: Location of squamocolum...
figure1.8: Location of squamocolum...
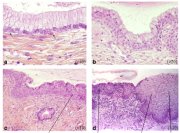 figure 1.9: Development of squamou...
figure 1.9: Development of squamou...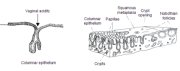 figure 1.10: Squamous metaplastic ...
figure 1.10: Squamous metaplastic ...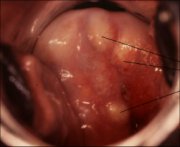 figure 1.11: Multiple nabothian cy...
figure 1.11: Multiple nabothian cy...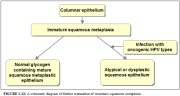 figure1.12: A schematic diagram o...
figure1.12: A schematic diagram o...
 figure1.7: Location of the squamoc...
figure1.7: Location of the squamoc...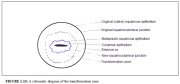 figure 1.13: A schematic diagram o...
figure 1.13: A schematic diagram o...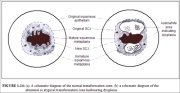 figure1.14: (a) A schematic diagra...
figure1.14: (a) A schematic diagra...
Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual, Edited by J.W. Sellors and R. Sankaranarayanan
Chapter 1: An introduction to the anatomy of the uterine cervix
Filter by language: English / Français / Español / Portugues / 中文- The cervix, the lower fibromuscular portion of the uterus, measures 3-4 cm in length and 2.5 cm in diameter; however, it varies in size and shape depending on age, parity and menstrual status of the woman.
- Ectocervix is the most readily visible portion of the cervix; endocervix is largely invisible and lies proximal to the external os.
- Ectocervix is covered by a pink stratified squamous epithelium, consisting of multiple layers of cells and a reddish columnar epithelium consisting of a single layer of cells lines the endocervix. The intermediate and superficial cell layers of the squamous epithelium contain glycogen.
- The location of squamocolumnar junction in relation to the external os varies depending upon age, menstrual status, and other factors such as pregnancy and oral contraceptive use.
- Ectropion refers to the eversion of the columnar epithelium onto the ectocervix, when the cervix grows rapidly and enlarges under the influence of estrogen, after menarche and during pregnancy.
- Squamous metaplasia in the cervix refers to the physiological replacement of the everted columnar epithelium on the ectocervix by a newly formed squamous epithelium from the subcolumnar reserve cells.
- The region of the cervix where squamous metaplasia occurs is referred to as the transformation zone.
- Identifying the transformation zone is of great importance in colposcopy, as almost all manifestations of cervical carcinogenesis occur in this zone.
A thorough understanding of the anatomy and physiology of the cervix is absolutely essential for effective colposcopic practice. This chapter deals with the gross and microscopic anatomy of the uterine cervix and the physiology of the transformation zone. The cervix is the lower fibromuscular portion of the uterus. It is cylindrical or conical in shape, and measures 3 to 4 cm in length, and 2.5 cm in diameter. It is supported by the cardinal and uterosacral ligaments, which stretch between the lateral and posterior portions of the cervix and the walls of the bony pelvis. The lower half of the cervix, called the portio vaginalis, protrudes into the vagina through its anterior wall, and the upper half remains above the vagina (Figure 1.1). The portio vaginalis opens into the vagina through an orifice called the external os.
The cervix varies in size and shape depending on the woman’s age, parity and hormonal status. In parous women, it is bulky and the external os appears as a wide, gaping, transverse slit. In nulliparous women, the external os resembles a small circular opening in the centre of the cervix. The supravaginal portion meets with the muscular body of the uterus at the internal cervical os. The portion of the cervix lying exterior to the external os is called the ectocervix. This is the portion of the cervix that is readily visible on speculum examination. The portion proximal to the external os is called the endocervix and the external os needs to be stretched or dilated to view this portion of the cervix. The endocervical canal, which traverses the endocervix, connects the uterine cavity with the vagina and extends from the internal to the external os, where it opens into the vagina. It varies in length and width depending on the woman’s age and hormonal status. It is widest in women in the reproductive age group, when it measures 6-8 mm in width.
The space surrounding the cervix in the vaginal cavity is called the vaginal fornix. The part of the fornix between the cervix and the lateral vaginal walls is called the lateral fornix; the portions between the anterior and posterior walls of the vagina and the cervix are termed the anterior and posterior fornix, respectively.
The stroma of the cervix is composed of dense, fibro-muscular tissue through which vascular, lymphatic and nerve supplies to the cervix pass and form a complex plexus. The arterial supply of the cervix is derived from internal iliac arteries through the cervical and vaginal branches of the uterine arteries. The cervical branches of the uterine arteries descend in the lateral aspects of the cervix at 3 and 9 o’clock positions. The veins of the cervix run parallel to the arteries and drain into the hypogastric venous plexus. The lymphatic vessels from the cervix drain into the common, external and internal iliac nodes, obturator and the parametrial nodes. The nerve supply to the cervix is derived from the hypogastric plexus. The endocervix has extensive sensory nerve endings, while there are very few in the ectocervix. Hence, procedures such as biopsy, electrocoagulation and cryotherapy are well tolerated in most women without local anaesthesia. Since sympathetic and parasympathetic fibres are also abundant in the endocervix, dilatation and curettage of the endocervix may occasionally lead to a vasovagal reaction.
The cervix is covered by both stratified non-keratinizing squamous and columnar epithelium. These two types of epithelium meet at the squamocolumnar junction.
The cervix varies in size and shape depending on the woman’s age, parity and hormonal status. In parous women, it is bulky and the external os appears as a wide, gaping, transverse slit. In nulliparous women, the external os resembles a small circular opening in the centre of the cervix. The supravaginal portion meets with the muscular body of the uterus at the internal cervical os. The portion of the cervix lying exterior to the external os is called the ectocervix. This is the portion of the cervix that is readily visible on speculum examination. The portion proximal to the external os is called the endocervix and the external os needs to be stretched or dilated to view this portion of the cervix. The endocervical canal, which traverses the endocervix, connects the uterine cavity with the vagina and extends from the internal to the external os, where it opens into the vagina. It varies in length and width depending on the woman’s age and hormonal status. It is widest in women in the reproductive age group, when it measures 6-8 mm in width.
The space surrounding the cervix in the vaginal cavity is called the vaginal fornix. The part of the fornix between the cervix and the lateral vaginal walls is called the lateral fornix; the portions between the anterior and posterior walls of the vagina and the cervix are termed the anterior and posterior fornix, respectively.
The stroma of the cervix is composed of dense, fibro-muscular tissue through which vascular, lymphatic and nerve supplies to the cervix pass and form a complex plexus. The arterial supply of the cervix is derived from internal iliac arteries through the cervical and vaginal branches of the uterine arteries. The cervical branches of the uterine arteries descend in the lateral aspects of the cervix at 3 and 9 o’clock positions. The veins of the cervix run parallel to the arteries and drain into the hypogastric venous plexus. The lymphatic vessels from the cervix drain into the common, external and internal iliac nodes, obturator and the parametrial nodes. The nerve supply to the cervix is derived from the hypogastric plexus. The endocervix has extensive sensory nerve endings, while there are very few in the ectocervix. Hence, procedures such as biopsy, electrocoagulation and cryotherapy are well tolerated in most women without local anaesthesia. Since sympathetic and parasympathetic fibres are also abundant in the endocervix, dilatation and curettage of the endocervix may occasionally lead to a vasovagal reaction.
The cervix is covered by both stratified non-keratinizing squamous and columnar epithelium. These two types of epithelium meet at the squamocolumnar junction.
 figure 1.1: Gross anatomy of the u...
figure 1.1: Gross anatomy of the u...Stratified non-keratinizing squamous epithelium
Normally, a large area of ectocervix is covered by a stratified, non-keratinizing, glycogen-containing squamous epithelium. It is opaque, has multiple (15-20) layers of cells (Figure 1.2) and is pale pink in colour. This epithelium may be native to the site formed during embryonic life, which is called the native or original squamous epithelium, or it may have been newly formed as metaplastic squamous epithelium in early adult life. In premenopausal women, the original squamous epithelium is pinkish in colour, whereas the newly formed metaplastic squamous epithelium looks somewhat pinkish-white on visual examination.
The histological architecture of the squamous epithelium of the cervix reveals, at the bottom, a single layer of round basal cells with a large dark-staining nuclei and little cytoplasm, attached to the basement membrane (Figure 1.2). The basement membrane separates the epithelium from the underlying stroma. The epithelial-stromal junction is usually straight. Sometimes it is slightly undulating with short projections of stroma at regular intervals. These stromal projections are called papillae. The parts of the epithelium between the papillae are called rete pegs.
The basal cells divide and mature to form the next few layers of cells called parabasal cells, which also have relatively large dark-staining nuclei and greenish-blue basophilic cytoplasm. Further differentiation and maturation of these cells leads to the intermediate layers of polygonal cells with abundant cytoplasm and small round nuclei. These cells form a basket-weave pattern. With further maturation, the large and markedly flattened cells with small, dense, pyknotic nuclei and transparent cytoplasm of the superficial layers are formed. Overall, from the basal to the superficial layer, these cells undergo an increase in size and a reduction of nuclear size.
The intermediate and superficial layer cells contain abundant glycogen in their cytoplasm, which stains mahogany brown or black after application of Lugol’s iodine and magenta with periodic acid-Schiff stain in histological sections. Glycogenation of the intermediate and superficial layers is a sign of normal maturation and development of the squamous epithelium. Abnormal or altered maturation is characterized by a lack of glycogen production.
The maturation of the squamous epithelium of the cervix is dependent on estrogen, the female hormone. If estrogen is lacking, full maturation and glycogenation does not take place. Hence, after menopause, the cells do not mature beyond the parabasal layer and do not accumulate as multiple layers of flat cells. Consequently, the epithelium becomes thin and atrophic. On visual examination, it appears pale, with subepithelial petechial haemorrhagic spots, as it is easily prone to trauma.
The histological architecture of the squamous epithelium of the cervix reveals, at the bottom, a single layer of round basal cells with a large dark-staining nuclei and little cytoplasm, attached to the basement membrane (Figure 1.2). The basement membrane separates the epithelium from the underlying stroma. The epithelial-stromal junction is usually straight. Sometimes it is slightly undulating with short projections of stroma at regular intervals. These stromal projections are called papillae. The parts of the epithelium between the papillae are called rete pegs.
The basal cells divide and mature to form the next few layers of cells called parabasal cells, which also have relatively large dark-staining nuclei and greenish-blue basophilic cytoplasm. Further differentiation and maturation of these cells leads to the intermediate layers of polygonal cells with abundant cytoplasm and small round nuclei. These cells form a basket-weave pattern. With further maturation, the large and markedly flattened cells with small, dense, pyknotic nuclei and transparent cytoplasm of the superficial layers are formed. Overall, from the basal to the superficial layer, these cells undergo an increase in size and a reduction of nuclear size.
The intermediate and superficial layer cells contain abundant glycogen in their cytoplasm, which stains mahogany brown or black after application of Lugol’s iodine and magenta with periodic acid-Schiff stain in histological sections. Glycogenation of the intermediate and superficial layers is a sign of normal maturation and development of the squamous epithelium. Abnormal or altered maturation is characterized by a lack of glycogen production.
The maturation of the squamous epithelium of the cervix is dependent on estrogen, the female hormone. If estrogen is lacking, full maturation and glycogenation does not take place. Hence, after menopause, the cells do not mature beyond the parabasal layer and do not accumulate as multiple layers of flat cells. Consequently, the epithelium becomes thin and atrophic. On visual examination, it appears pale, with subepithelial petechial haemorrhagic spots, as it is easily prone to trauma.
 figure 1.2: Stratified squamous ep...
figure 1.2: Stratified squamous ep...Columnar epithelium
The endocervical canal is lined by the columnar epithelium (sometimes referred to as glandular epithelium). It is composed of a single layer of tall cells with dark-staining nuclei close to the basement membrane (Figure 1.3). Because of its single layer of cells, it is much shorter in height than the stratified squamous epithelium of the cervix. On visual examination, it appears reddish in colour because the thin single cell layer allows the coloration of the underlying vasculature in the stroma to be seen more easily. At its distal or upper limit, it merges with the endometrial epithelium in the lower part of the body of the uterus. At its proximal or lower limit, it meets with the squamous epithelium at the squamocolumnar junction. It covers a variable extent of the ectocervix, depending upon the woman’s age, reproductive, hormonal and menopausal status.
The columnar epithelium does not form a flattened surface in the cervical canal, but is thrown into multiple longitudinal folds protruding into the lumen of the canal, giving rise to papillary projections. It forms several invaginations into the substance of the cervical stroma, resulting in the formation of endocervical crypts (sometimes referred to as endocervical glands) (Figure 1.4). The crypts may traverse as far as 5-8 mm from the surface of the cervix. This complex architecture, consisting of mucosal folds and crypts, gives the columnar epithelium a grainy appearance on visual inspection.
A localized overgrowth of the endocervical columnar epithelium may occasionally be visible as a reddish mass protruding from the external os on visual examination of the cervix. This is called a cervical polyp (Figure 1.5). It usually begins as a localized enlargement of a single columnar papilla and appears as a mass as it enlarges. It is composed of a core of endocervical stroma lined by the columnar epithelium with underlying crypts. Occasionally, multiple polyps may arise from the columnar epithelium.
Glycogenation and mitoses are absent in the columnar epithelium. Because of the lack of intracytoplasmic glycogen, the columnar epithelium does not change colour after the application of Lugol’s iodine or remains slightly discoloured with a thin film of iodine solution.
The columnar epithelium does not form a flattened surface in the cervical canal, but is thrown into multiple longitudinal folds protruding into the lumen of the canal, giving rise to papillary projections. It forms several invaginations into the substance of the cervical stroma, resulting in the formation of endocervical crypts (sometimes referred to as endocervical glands) (Figure 1.4). The crypts may traverse as far as 5-8 mm from the surface of the cervix. This complex architecture, consisting of mucosal folds and crypts, gives the columnar epithelium a grainy appearance on visual inspection.
A localized overgrowth of the endocervical columnar epithelium may occasionally be visible as a reddish mass protruding from the external os on visual examination of the cervix. This is called a cervical polyp (Figure 1.5). It usually begins as a localized enlargement of a single columnar papilla and appears as a mass as it enlarges. It is composed of a core of endocervical stroma lined by the columnar epithelium with underlying crypts. Occasionally, multiple polyps may arise from the columnar epithelium.
Glycogenation and mitoses are absent in the columnar epithelium. Because of the lack of intracytoplasmic glycogen, the columnar epithelium does not change colour after the application of Lugol’s iodine or remains slightly discoloured with a thin film of iodine solution.
 figure 1.3: Columnar epithelium (x...
figure 1.3: Columnar epithelium (x... figure 1.4: Crypts of columnar epi...
figure 1.4: Crypts of columnar epi... figure 1.5: Cervical polyp
figure 1.5: Cervical polyp...
Squamocolumnar junction
The squamocolumnar junction (Figures 1.6 and 1.7) appears as a sharp line with a step, due to the difference in the height of the squamous and columnar epithelium. The location of the squamocolumnar junction in relation to the external os is variable over a woman’s lifetime and depends upon factors such as age, hormonal status, birth trauma, oral contraceptive use and certain physiological conditions such as pregnancy (Figures 1.6 and 1.7).
The squamocolumnar junction visible during childhood, perimenarche, after puberty and early reproductive period is referred to as the original squamocolumnar junction, as this represents the junction between the columnar epithelium and the ‘original’ squamous epithelium laid down during embryogenesis and intrauterine life. During childhood and perimenarche, the original squamocolumnar junction is located at, or very close to, the external os (Figure 1.7a). After puberty and during the reproductive period, the female genital organs grow under the influence of estrogen. Thus, the cervix swells and enlarges and the endocervical canal elongates. This leads to the eversion of the columnar epithelium of the lower part of the endocervical canal on to the ectocervix (Figure 1.7b). This condition is called ectropion or ectopy, which is visible as a strikingly reddish-looking ectocervix on visual inspection (Figure 1.8a). It is sometimes called ‘erosion’ or ‘ulcer’, which are misnomers and should not be used to denote this condition. Thus the original squamocolumnar junction is located on the ectocervix, far away from the external os (Figures 1.7b and 1.8a). Ectropion becomes much more pronounced during pregnancy.
The buffer action of the mucus covering the columnar cells is interfered with when the everted columnar epithelium in ectropion is exposed to the acidic vaginal environment. This leads to the destruction and eventual replacement of the columnar epithelium by the newly formed metaplastic squamous epithelium. Metaplasia refers to the change or replacement of one type of epithelium by another.
The metaplastic process mostly starts at the original squamocolumnar junction and proceeds centripetally towards the external os through the reproductive period to perimenopause. Thus, a new squamocolumnar junction is formed between the newly formed metaplastic squamous epithelium and the columnar epithelium remaining everted onto the ectocervix (Figures 1.7c, 1.8b). As the woman passes from the reproductive to the perimenopausal age group, the location of the new squamocolumnar junction progressively moves on the ectocervix towards the external os (Figures 1.7c, 1.7d, 1.7e and 1.8). Hence, it is located at variable distances from the external os, as a result of the progressive formation of the new metaplastic squamous epithelium in the exposed areas of the columnar epithelium in the ectocervix. From the perimenopausal period and after the onset of menopause, the cervix shrinks due the lack of estrogen, and consequently, the movement of the new squamocolumnar junction towards the external os and into the endocervical canal is accelerated (Figures 1.7d and 1.8c). In postmenopausal women, the new squamocolumnar junction is often invisible on visual examination (Figures 1.7e and 1.8d).
The new squamocolumnar junction is hereafter simply referred to as squamocolumnar junction in this manual. Reference to the original squamocolumnar junction will be explicitly made as the original squamocolumnar junction.
The squamocolumnar junction visible during childhood, perimenarche, after puberty and early reproductive period is referred to as the original squamocolumnar junction, as this represents the junction between the columnar epithelium and the ‘original’ squamous epithelium laid down during embryogenesis and intrauterine life. During childhood and perimenarche, the original squamocolumnar junction is located at, or very close to, the external os (Figure 1.7a). After puberty and during the reproductive period, the female genital organs grow under the influence of estrogen. Thus, the cervix swells and enlarges and the endocervical canal elongates. This leads to the eversion of the columnar epithelium of the lower part of the endocervical canal on to the ectocervix (Figure 1.7b). This condition is called ectropion or ectopy, which is visible as a strikingly reddish-looking ectocervix on visual inspection (Figure 1.8a). It is sometimes called ‘erosion’ or ‘ulcer’, which are misnomers and should not be used to denote this condition. Thus the original squamocolumnar junction is located on the ectocervix, far away from the external os (Figures 1.7b and 1.8a). Ectropion becomes much more pronounced during pregnancy.
The buffer action of the mucus covering the columnar cells is interfered with when the everted columnar epithelium in ectropion is exposed to the acidic vaginal environment. This leads to the destruction and eventual replacement of the columnar epithelium by the newly formed metaplastic squamous epithelium. Metaplasia refers to the change or replacement of one type of epithelium by another.
The metaplastic process mostly starts at the original squamocolumnar junction and proceeds centripetally towards the external os through the reproductive period to perimenopause. Thus, a new squamocolumnar junction is formed between the newly formed metaplastic squamous epithelium and the columnar epithelium remaining everted onto the ectocervix (Figures 1.7c, 1.8b). As the woman passes from the reproductive to the perimenopausal age group, the location of the new squamocolumnar junction progressively moves on the ectocervix towards the external os (Figures 1.7c, 1.7d, 1.7e and 1.8). Hence, it is located at variable distances from the external os, as a result of the progressive formation of the new metaplastic squamous epithelium in the exposed areas of the columnar epithelium in the ectocervix. From the perimenopausal period and after the onset of menopause, the cervix shrinks due the lack of estrogen, and consequently, the movement of the new squamocolumnar junction towards the external os and into the endocervical canal is accelerated (Figures 1.7d and 1.8c). In postmenopausal women, the new squamocolumnar junction is often invisible on visual examination (Figures 1.7e and 1.8d).
The new squamocolumnar junction is hereafter simply referred to as squamocolumnar junction in this manual. Reference to the original squamocolumnar junction will be explicitly made as the original squamocolumnar junction.
 figure 1.6: Squamocolumnar junctio...
figure 1.6: Squamocolumnar junctio... figure 1.7: Location of the squamo...
figure 1.7: Location of the squamo... figure 1.8: Location of squamocolu...
figure 1.8: Location of squamocolu...Ectropion or ectopy
Ectropion or ectopy is defined as the presence of everted endocervical columnar epithelium on the ectocervix. It appears as a large reddish area on the ectocervix surrounding the external os (Figures 1.7b and 1.8a). The eversion of the columnar epithelium is more pronounced on the anterior and posterior lips of the ectocervix and less on the lateral lips. This is a normal, physiological occurrence in a woman’s life. Occasionally the columnar epithelium extends into the vaginal fornix. The whole mucosa including the crypts and the supporting stroma is displaced in ectropion. It is the region in which physiological transformation to squamous metaplasia, as well as abnormal transformation in cervical carcinogenesis, occurs.
 figure1.7: Location of the squamoc...
figure1.7: Location of the squamoc... figure1.8: Location of squamocolum...
figure1.8: Location of squamocolum...Squamous metaplasia
The physiological replacement of the everted columnar epithelium by a newly formed squamous epithelium is called squamous metaplasia. The vaginal environment is acidic during the reproductive years and during pregnancy. The acidity is thought to play a role in squamous metaplasia. When the cells are repeatedly destroyed by vaginal acidity in the columnar epithelium in an area of ectropion, they are eventually replaced by a newly formed metaplastic epithelium. The irritation of exposed columnar epithelium by the acidic vaginal environment results in the appearance of sub-columnar reserve cells. These cells proliferate producing a reserve cell hyperplasia and eventually form the metaplastic squamous epithelium.
As already indicated, the metaplastic process requires the appearance of undifferentiated, cuboidal, sub-columnar cells called reserve cells (Figure 1.9a), for the metaplastic squamous epithelium is produced from the multiplication and differentiation of these cells. These eventually lift off the persistent columnar epithelium (Figures 1.9b and 1.9c). The exact origin of the reserve cells is not known, though it is widely believed that it develops from the columnar epithelium, in response to irritation by the vaginal acidity.
The first sign of squamous metaplasia is the appearance and proliferation of reserve cells (Figures 1.9a and 1.9b). This is initially seen as a single layer of small, round cells with darkly staining nuclei, situated very close to the nuclei of columnar cells, which further proliferate to produce a reserve cell hyperplasia (Figure 1.9b). Morphologically, the reserve cells have a similar appearance to the basal cells of the original squamous epithelium, with round nuclei and little cytoplasm. As the metaplastic process progresses, the reserve cells proliferate and differentiate to form a thin, multicellular epithelium of immature squamous cells with no evidence of stratification (Figure 1.9c). The term immature squamous metaplastic epithelium is applied when there is little or no stratification in this thin newly formed metaplastic epithelium. The cells in the immature squamous metaplastic epithelium do not produce glycogen and, hence, do not stain brown or black with Lugol’s iodine solution. Groups of mucin-containing columnar cells may be seen embedded in the immature squamous metaplastic epithelium at this stage.
Numerous continuous and/or isolated fields or foci of immature squamous metaplasia may arise at the same time. It has been proposed that the basement membrane of the original columnar epithelium dissolves and is reformed between the proliferating and differentiating reserve cells and the cervical stroma. Squamous metaplasia usually begins at the original squamocolumnar junction at the distal limit of the ectopy, but it may also occur in the columnar epithelium close to this junction or as islands scattered in the exposed columnar epithelium.
As the process continues, the immature metaplastic squamous cells differentiate into mature stratified metaplastic epithelium (Figure 1.9d). For all practical purposes, the latter resembles the original stratified squamous epithelium. Some residual columnar cells or vacuoles of mucus are seen in the mature squamous metaplastic epithelium, which contains glycogen from the intermediate cell layer onwards. Thus, it stains brown or black after application of Lugol’s iodine. Several cysts, called nabothian cysts (follicles), may be observed in the mature metaplastic squamous epithelium (1.10 and 1.11). Nabothian cysts are retention cysts that develop as a result of the occlusion of an endocervical crypt opening or outlet by the overlying metaplastic squamous epithelium (1.10). The buried columnar epithelium continues to secrete mucus, which eventually fills and distends the cyst. The entrapped mucus gives an ivory-white to yellowish hue to the cyst on visual examination (1.11). The columnar epithelium in the wall of the cyst is flattened and ultimately destroyed by the pressure of the mucus in it. The outlets of the crypts of columnar epithelium, not yet covered by the metaplastic epithelium, remain as persistent crypt openings. The farthest extent of the metaplastic epithelium onto the ectocervix can be best judged by the location of the crypt opening farthest away from the squamocolumnar junction.
Squamous metaplasia is an irreversible process; the transformed epithelium (now squamous in character) cannot revert to columnar epithelium. The metaplastic process in the cervix is sometimes referred to as indirect metaplasia, as the columnar cells do not transform into squamous cells, but are replaced by the proliferating sub-columnar cuboidal reserve cells. Squamous metaplasia may progress at varying rates in different areas of the same cervix, and hence many areas of widely differing maturity may be seen in the metaplastic squamous epithelium with or without islands of columnar epithelium. The metaplastic epithelium adjacent to the squamocolumnar junction is composed of immature metaplasia, and the mature metaplastic epithelium is found near the original squamocolumnar junction.
Further development of the newly formed immature metaplastic epithelium may take two directions (1.12). In the vast majority of women, it develops into a mature squamous metaplastic epithelium, which is similar to the normal glycogen-containing original squamous epithelium for all practical purposes. In a very small minority of women, an atypical, dysplastic epithelium may develop. Certain oncogenic human papillomavirus (HPV) types may persistently infect the immature basal squamous metaplastic cells and transform them into atypical cells with nuclear and cytoplasmic abnormalities. The uncontrolled proliferation and expansion of these atypical cells may lead to the formation of an abnormal dysplastic epithelium which may regress to normal, persist as dysplasia or progress into invasive cancer after several years.
It is also thought that some metaplasia may occur by in-growth of the squamous epithelium from the squamous epithelium of the ectocervix.
As already indicated, the metaplastic process requires the appearance of undifferentiated, cuboidal, sub-columnar cells called reserve cells (Figure 1.9a), for the metaplastic squamous epithelium is produced from the multiplication and differentiation of these cells. These eventually lift off the persistent columnar epithelium (Figures 1.9b and 1.9c). The exact origin of the reserve cells is not known, though it is widely believed that it develops from the columnar epithelium, in response to irritation by the vaginal acidity.
The first sign of squamous metaplasia is the appearance and proliferation of reserve cells (Figures 1.9a and 1.9b). This is initially seen as a single layer of small, round cells with darkly staining nuclei, situated very close to the nuclei of columnar cells, which further proliferate to produce a reserve cell hyperplasia (Figure 1.9b). Morphologically, the reserve cells have a similar appearance to the basal cells of the original squamous epithelium, with round nuclei and little cytoplasm. As the metaplastic process progresses, the reserve cells proliferate and differentiate to form a thin, multicellular epithelium of immature squamous cells with no evidence of stratification (Figure 1.9c). The term immature squamous metaplastic epithelium is applied when there is little or no stratification in this thin newly formed metaplastic epithelium. The cells in the immature squamous metaplastic epithelium do not produce glycogen and, hence, do not stain brown or black with Lugol’s iodine solution. Groups of mucin-containing columnar cells may be seen embedded in the immature squamous metaplastic epithelium at this stage.
Numerous continuous and/or isolated fields or foci of immature squamous metaplasia may arise at the same time. It has been proposed that the basement membrane of the original columnar epithelium dissolves and is reformed between the proliferating and differentiating reserve cells and the cervical stroma. Squamous metaplasia usually begins at the original squamocolumnar junction at the distal limit of the ectopy, but it may also occur in the columnar epithelium close to this junction or as islands scattered in the exposed columnar epithelium.
As the process continues, the immature metaplastic squamous cells differentiate into mature stratified metaplastic epithelium (Figure 1.9d). For all practical purposes, the latter resembles the original stratified squamous epithelium. Some residual columnar cells or vacuoles of mucus are seen in the mature squamous metaplastic epithelium, which contains glycogen from the intermediate cell layer onwards. Thus, it stains brown or black after application of Lugol’s iodine. Several cysts, called nabothian cysts (follicles), may be observed in the mature metaplastic squamous epithelium (1.10 and 1.11). Nabothian cysts are retention cysts that develop as a result of the occlusion of an endocervical crypt opening or outlet by the overlying metaplastic squamous epithelium (1.10). The buried columnar epithelium continues to secrete mucus, which eventually fills and distends the cyst. The entrapped mucus gives an ivory-white to yellowish hue to the cyst on visual examination (1.11). The columnar epithelium in the wall of the cyst is flattened and ultimately destroyed by the pressure of the mucus in it. The outlets of the crypts of columnar epithelium, not yet covered by the metaplastic epithelium, remain as persistent crypt openings. The farthest extent of the metaplastic epithelium onto the ectocervix can be best judged by the location of the crypt opening farthest away from the squamocolumnar junction.
Squamous metaplasia is an irreversible process; the transformed epithelium (now squamous in character) cannot revert to columnar epithelium. The metaplastic process in the cervix is sometimes referred to as indirect metaplasia, as the columnar cells do not transform into squamous cells, but are replaced by the proliferating sub-columnar cuboidal reserve cells. Squamous metaplasia may progress at varying rates in different areas of the same cervix, and hence many areas of widely differing maturity may be seen in the metaplastic squamous epithelium with or without islands of columnar epithelium. The metaplastic epithelium adjacent to the squamocolumnar junction is composed of immature metaplasia, and the mature metaplastic epithelium is found near the original squamocolumnar junction.
Further development of the newly formed immature metaplastic epithelium may take two directions (1.12). In the vast majority of women, it develops into a mature squamous metaplastic epithelium, which is similar to the normal glycogen-containing original squamous epithelium for all practical purposes. In a very small minority of women, an atypical, dysplastic epithelium may develop. Certain oncogenic human papillomavirus (HPV) types may persistently infect the immature basal squamous metaplastic cells and transform them into atypical cells with nuclear and cytoplasmic abnormalities. The uncontrolled proliferation and expansion of these atypical cells may lead to the formation of an abnormal dysplastic epithelium which may regress to normal, persist as dysplasia or progress into invasive cancer after several years.
It is also thought that some metaplasia may occur by in-growth of the squamous epithelium from the squamous epithelium of the ectocervix.
 figure 1.9: Development of squamou...
figure 1.9: Development of squamou... figure 1.10: Squamous metaplastic ...
figure 1.10: Squamous metaplastic ... figure 1.11: Multiple nabothian cy...
figure 1.11: Multiple nabothian cy... figure1.12: A schematic diagram o...
figure1.12: A schematic diagram o...Transformation zone
This region of the cervix where the columnar epithelium has been replaced and/or is being replaced by the new metaplastic squamous epithelium is referred to as the transformation zone. It corresponds to the area of cervix bound by the original squamocolumnar junction at the distal end and proximally by the furthest extent that squamous metaplasia has occurred as defined by the new squamocolumnar junction (Figures 1.7, 1.13 and 1.14). In premenopausal women, the transformation zone is fully located on the ectocervix. After menopause through old age, the cervix shrinks with the decreasing levels of estrogen. Consequently, the transformation zone may move partially, and later fully, into the cervical canal.
The transformation zone may be described as normal when it is composed of immature and/or mature squamous metaplasia along with intervening areas or islands of columnar epithelium, with no signs of cervical carcinogenesis (1.14a). It is termed an abnormal or atypical transformation zone (ATZ) when evidence of cervical carcinogenesis such as dysplastic change is observed in the transformation zone (1.14b). Identifying the transformation zone is of great importance in colposcopy, as almost all manifestations of cervical carcinogenesis occur in this zone.
The transformation zone may be described as normal when it is composed of immature and/or mature squamous metaplasia along with intervening areas or islands of columnar epithelium, with no signs of cervical carcinogenesis (1.14a). It is termed an abnormal or atypical transformation zone (ATZ) when evidence of cervical carcinogenesis such as dysplastic change is observed in the transformation zone (1.14b). Identifying the transformation zone is of great importance in colposcopy, as almost all manifestations of cervical carcinogenesis occur in this zone.
 figure1.7: Location of the squamoc...
figure1.7: Location of the squamoc... figure 1.13: A schematic diagram o...
figure 1.13: A schematic diagram o... figure1.14: (a) A schematic diagra...
figure1.14: (a) A schematic diagra...Congenital transformation zone
During early embryonic life, the cuboidal epithelium of the vaginal tube is replaced by the squamous epithelium, which begins at the caudal end of the dorsal urogenital sinus. This process is completed well before birth and the entire length of vagina and the ectocervix is meant to be covered by squamous epithelium. This process proceeds very rapidly along the lateral walls, and later in the anterior and posterior vaginal walls. If the epithelialization proceeds normally, the original squamocolumnar junction will be located at the external os at birth. On the other hand, if this process is arrested for some reason or incomplete, the original squamocolumnar junction will be located distal to the external os or may rarely be located on the vaginal walls, particularly involving the anterior and posterior fornices. The cuboidal epithelium remaining here will undergo squamous metaplasia. This late conversion to squamous epithelium in the anterior and posterior vaginal walls, as well as the ectocervix, results in the formation of the congenital transformation zone. Thus, it is a variant of intrauterine squamous metaplasia, in which differentiation of the squamous epithelium is not fully completed due to an interference with normal maturation. Excessive maturation is seen on the surface (as evidenced by keratinization) with delayed, incomplete maturation in deeper layers. Clinically, it may be seen as an extensive whitish-grey, hyperkeratotic area extending from the anterior and posterior lips of the cervix to the vaginal fornices. Gradual maturation of the epithelium may occur over several years. This type of transformation zone is seen in less than 5 % of women and is a variant of the normal transformation zone.



