Home / Training / Manuals / Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual / Chapter 7: Colposcopic assessment of cervical intraepithelial neoplasia
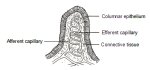 figure 6.4: Capillary network in c...
figure 6.4: Capillary network in c... FIGURE 2.3: Histology of CIN 2: At...
FIGURE 2.3: Histology of CIN 2: At... FIGURE 2.4: Histology of CIN 3: Dy...
FIGURE 2.4: Histology of CIN 3: Dy...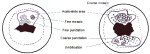 figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a...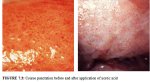 figure 7.3: Coarse punctation befo...
figure 7.3: Coarse punctation befo...
 figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a...
 figure 7.3: Coarse punctation befo...
figure 7.3: Coarse punctation befo... figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a...
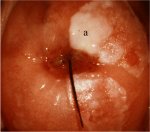 figure 7.4: Hyperkeratosis (leukop...
figure 7.4: Hyperkeratosis (leukop...
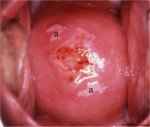 figure 7.5: The geographic satelli...
figure 7.5: The geographic satelli...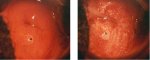 figure 7.6: Exophytic condyloma in...
figure 7.6: Exophytic condyloma in...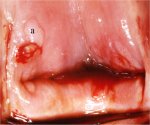 figure 7.7: Exophytic condyloma in...
figure 7.7: Exophytic condyloma in...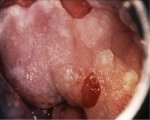 figure 7.8: Condyloma with an ence...
figure 7.8: Condyloma with an ence...
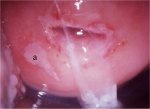 figure 7.9: Geographic satellite l...
figure 7.9: Geographic satellite l...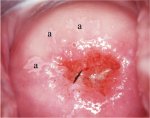 figure 7.10: Geographic satellite ...
figure 7.10: Geographic satellite ...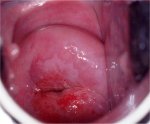 figure 7.11: Thin acetowhite lesio...
figure 7.11: Thin acetowhite lesio...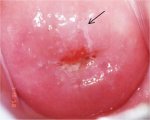 figure 7.12: Mildly dense, thin, e...
figure 7.12: Mildly dense, thin, e...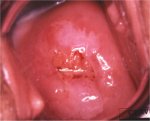 figure 7.13: Mildly dense acetowhi...
figure 7.13: Mildly dense acetowhi...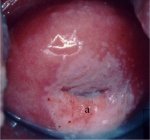 figure 7.14: Note the circumorific...
figure 7.14: Note the circumorific...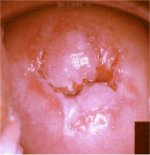 figure 7.15: Moderately dense acet...
figure 7.15: Moderately dense acet...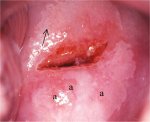 figurec7.16: Circumorificial, mild...
figurec7.16: Circumorificial, mild...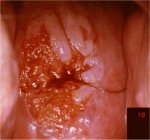 figure 7.17: Moderately dense acet...
figure 7.17: Moderately dense acet...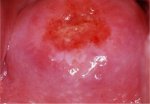 figure 7.18: Dense, well defined a...
figure 7.18: Dense, well defined a...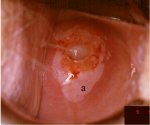 figure7.19: A dense acetowhite les...
figure7.19: A dense acetowhite les...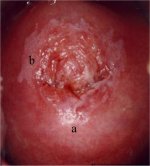 figure 7.20: Acetowhite lesions wi...
figure 7.20: Acetowhite lesions wi...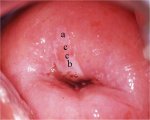 figure 7.21: An acetowhite lesion ...
figure 7.21: An acetowhite lesion ...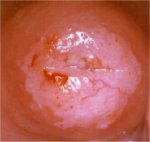 figure 7.22: A circumorificial den...
figure 7.22: A circumorificial den...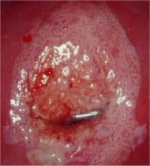 figure 7.23: A dense acetowhite le...
figure 7.23: A dense acetowhite le...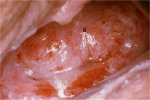 figure 7.24: Coarse mosaics (a) in...
figure 7.24: Coarse mosaics (a) in...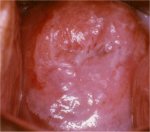 figure 7.25: Note the intensely de...
figure 7.25: Note the intensely de...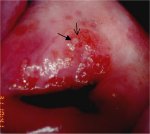 figure 7.26: A dense acetowhite le...
figure 7.26: A dense acetowhite le...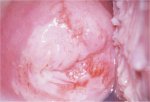 figure 7.27: A dense acetowhite, o...
figure 7.27: A dense acetowhite, o...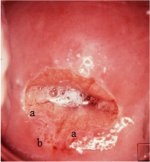 figure 6.11: The prominent white l...
figure 6.11: The prominent white l...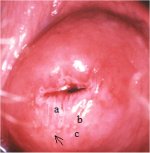 figure 6.12: Appearance after 5% a...
figure 6.12: Appearance after 5% a...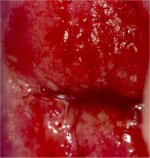 figure 9.2: Chronic cervicitis: th...
figure 9.2: Chronic cervicitis: th...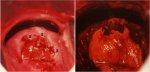 figure 9.5: Colposcopic appearance...
figure 9.5: Colposcopic appearance...
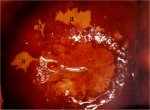 figure 7.28: Satellite lesions (a)...
figure 7.28: Satellite lesions (a)...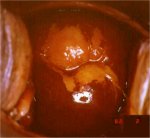 figure 7.29: A CIN 1 lesion with a...
figure 7.29: A CIN 1 lesion with a...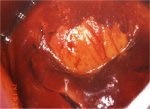 figure 7.30: Mustard yellow iodine...
figure 7.30: Mustard yellow iodine... figure 7.31: Dense saffron yellow ...
figure 7.31: Dense saffron yellow ...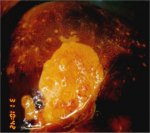 figure 7.32: A dense mustard yello...
figure 7.32: A dense mustard yello...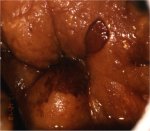 figure 7.33: A condylomatous lesio...
figure 7.33: A condylomatous lesio...
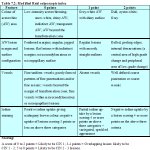 table 7.2: Modified Reid colposcop...
table 7.2: Modified Reid colposcop... table 7.3: Grading abnormal colpos...
table 7.3: Grading abnormal colpos...
Colposcopy and treatment of cervical intraepithelial neoplasia: a beginners’ manual, Edited by J.W. Sellors and R. Sankaranarayanan
Chapter 7: Colposcopic assessment of cervical intraepithelial neoplasia
Filter by language: English / Français / Español / Portugues / 中文- The colposcopic diagnosis of cervical neoplasia depends on the recognition of four main features: intensity (colour tone) of acetowhitening, margins and surface contour of acetowhite areas, vascular features and colour changes after iodine application.
- The occurrence of abnormal features in a localised area in the transformation zone increases the probability of diagnosis of a neoplastic lesion.
- Considerable skill may be required to differentiate between low-grade CIN, immature squamous metaplasia and inflammatory lesions.
- Biopsy should be directed whenever in doubt.
- The observation of well-demarcated, dense, opaque, acetowhite area(s) in the transformation zone close to or abutting the squamocolumnar junction is the hallmark of colposcopic diagnosis of CIN.
- Low-grade CIN is often seen as thin, smooth acetowhite lesions with well-demarcated, but irregular, feathery or digitating or angular margins.
- High-grade CIN are associated with thick, dense, dull, opaque or greyish-white acetowhite areas with well-demarcated, regular margins, which sometimes may be raised and rolled out. They may be more extensive and complex lesions extending into the endocervical canal. The surface contour of the acetowhite areas associated with high-grade CIN lesions tend to be less smooth, or irregular and nodular. Visualization of one or more borders within an acetowhite lesion or an acetowhite lesion with varying colour intensity is associated with high-grade lesions.
- Abnormal vascular features such as punctation and mosaics are significant only if these are seen confined to acetowhite areas.
- Vascular features, such as fine punctation and/or fine mosaics in acetowhite areas, may be associated with low-grade CIN.
- Coarse punctation and/or coarse mosaics in acetowhite areas tend to occur in high-grade lesions.
- CIN lesions do not contain glycogen and thus do not stain with iodine and remain mustard or saffron yellow areas.
- Using a scoring system such as Reid’s colposcopic index may guide colposcopic interpretation and diagnosis.
The colposcopic diagnosis of cervical neoplasia requires an understanding and recognition of four main features: colour tone and intensity of acetowhitening, margins and surface contour of acetowhite areas, vascular pattern and iodine staining. Colposcopy with directed biopsy is described as the reference investigation or ‘gold standard’ for the diagnosis of cervical precancer ( Singer & Monaghan, 2000). Colposcopy has a reported sensitivity ranging from 87% to 99% to diagnose cervical neoplasia, but its specificity is lower, between 23% and 87% ( Mitchell et al., 1998; Belinson et al., 2001).
The colposcopic features of cervical intraepithelial neoplasia (CIN) are described in this chapter to equip the student with the skills to distinguish the colposcopic findings associated with high-grade CIN (CIN 2-3) from those of low-grade lesions (CIN 1). Although the appearance of a single abnormal feature alone is not a strong indicator that a lesion is present, the occurrence of abnormal features together in a localized area in the transformation zone increases the probability of a lesion. It will become obvious during colposcopic practice that considerable skills are required to differentiate between low-grade lesions, immature squamous metaplasia and certain inflammatory conditions. The student is encouraged to obtain biopsies whenever in doubt, and to review the histopathological findings with the pathologist. Close collaboration with pathologists is obligatory and useful in improving one’s diagnostic skills. At the end of this chapter, a system that enables the colposcopist to score abnormalities is presented. This system is useful as a basis for the choice of which area(s) to select for biopsy. It is essential to biopsy the ‘worst’ area(s) - that is, the area(s) with the most severe changes in features.
The colposcopic findings of an abnormal or atypical transformation zone can involve the whole transformation zone but more commonly affect only a portion of it and there may be multiple distinct lesions. There is usually a distinct demarcation between normal and abnormal epithelium.
The colposcopic features that differentiate an abnormal transformation zone from the normal include the following: colour tone of acetowhite areas; surface pattern of acetowhite areas; borderline between acetowhite areas and the rest of the epithelium; vascular features and colour changes after application of iodine.
The colposcopic features of cervical intraepithelial neoplasia (CIN) are described in this chapter to equip the student with the skills to distinguish the colposcopic findings associated with high-grade CIN (CIN 2-3) from those of low-grade lesions (CIN 1). Although the appearance of a single abnormal feature alone is not a strong indicator that a lesion is present, the occurrence of abnormal features together in a localized area in the transformation zone increases the probability of a lesion. It will become obvious during colposcopic practice that considerable skills are required to differentiate between low-grade lesions, immature squamous metaplasia and certain inflammatory conditions. The student is encouraged to obtain biopsies whenever in doubt, and to review the histopathological findings with the pathologist. Close collaboration with pathologists is obligatory and useful in improving one’s diagnostic skills. At the end of this chapter, a system that enables the colposcopist to score abnormalities is presented. This system is useful as a basis for the choice of which area(s) to select for biopsy. It is essential to biopsy the ‘worst’ area(s) - that is, the area(s) with the most severe changes in features.
The colposcopic findings of an abnormal or atypical transformation zone can involve the whole transformation zone but more commonly affect only a portion of it and there may be multiple distinct lesions. There is usually a distinct demarcation between normal and abnormal epithelium.
The colposcopic features that differentiate an abnormal transformation zone from the normal include the following: colour tone of acetowhite areas; surface pattern of acetowhite areas; borderline between acetowhite areas and the rest of the epithelium; vascular features and colour changes after application of iodine.
After application of normal saline solution
Following application of saline, abnormal epithelium may appear much darker than the normal epithelium.
Vasculature
Using the green (or blue) filter and higher-power magnification when necessary, the best opportunity to evaluate any abnormal vasculature patterns is before the application of acetic acid, the effect of which may obscure some or all of the changes, especially in an acetowhite area. The abnormalities of interest are punctation, mosaics and atypical vessels.
Capillaries
The afferent and efferent capillaries within the villi (Figure 6.4) of columnar epithelium become compressed during the normal metaplastic process and are not incorporated within the newly formed squamous epithelium. Instead, they form a fine network below the basement membrane. When CIN develops as a result of HPV infection and atypical metaplasia, the afferent and efferent capillary system may be trapped (incorporated) into the diseased dysplastic epithelium through several elongated stromal papillae (Figures 2.3 and 2.4), and a thin layer of epithelium may remain on top of these vessels. This forms the basis of the punctate and mosaic blood vessel patterns (Figures 7.1, 7.2 and 7.3). The terminating vessels in the stromal papillae underlying the thin epithelium appear as black points in a stippling pattern in an end-on view under the colposcope, making what are called punctate areas (Figures 7.1, 7.2 and 7.3). The inter-connecting blood vessels in the stromal papillae surrounding the rete pegs of the epithelium, running parallel to the surface, are observed colposcopically as cobbled areas of mosaic pattern (Figures 7.1 and 7.2). In mosaic areas, the epithelium appears as individual small, large, round, polygonal, regular or irregular blocks. Punctation and mosaic areas may be classified as either fine or coarse. Coarse changes tend to be associated with more severe degrees of abnormality. When both punctation and mosaic patterns are found to coexist, the same evaluation criteria for colposcopic prediction of disease are used as when they exist separately.
Vessels exhibiting punctation and mosaics are usually more strikingly obvious than the normal stromal vessels because these vessels penetrate into the epithelium and are thus closer to the surface. When acetic acid is applied, these abnormal vascular patterns seen to be confined to the acetowhite areas.
Vessels exhibiting punctation and mosaics are usually more strikingly obvious than the normal stromal vessels because these vessels penetrate into the epithelium and are thus closer to the surface. When acetic acid is applied, these abnormal vascular patterns seen to be confined to the acetowhite areas.
 figure 6.4: Capillary network in c...
figure 6.4: Capillary network in c... FIGURE 2.3: Histology of CIN 2: At...
FIGURE 2.3: Histology of CIN 2: At... FIGURE 2.4: Histology of CIN 3: Dy...
FIGURE 2.4: Histology of CIN 3: Dy... figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a... figure 7.3: Coarse punctation befo...
figure 7.3: Coarse punctation befo...Fine punctation
Fine punctation refers to looped capillaries - viewed end-on - that appear to be of fine calibre and located close to one another, producing a delicate stippling effect (Figures 7.1 and 7.2a). Fine mosaics are a network of fine-calibre blood vessels that appear in close proximity to one another, as a mosaic pattern, when viewed with the colposcope (Figure 7.1). These two vascular appearances may occur together and may be found in low-grade (CIN 1) lesions. The patterns do not necessarily appear throughout the whole lesion.
 figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a...Coarse punctation and coarse mosaics
Coarse punctation (Figure 7.3) and coarse mosaics (Figures 7.1 and 7.2) are formed by vessels having larger calibre and larger intercapillary distances, in contrast to the corresponding fine changes. Coarse punctation and mosaicism tend to occur in more severe neoplastic lesions such as CIN 2, CIN 3 lesions and early preclinical invasive cancer. Sometimes, the two patterns are superimposed in an area so that the capillary loops occur in the centre of each mosaic ‘tile’. This appearance is called umbilication (Figure 7.1).
 figure 7.3: Coarse punctation befo...
figure 7.3: Coarse punctation befo... figure 7.1: A schematic representa...
figure 7.1: A schematic representa... figure 7.2a: Fine punctation (a) a...
figure 7.2a: Fine punctation (a) a...Leukoplakia (hyperkeratosis)
Leukoplakia or hyperkeratosis (Figure 7.4) is a white, well-demarcated area on the cervix that may be apparent to the unaided eye, before the application of acetic acid. The white colour is due to the presence of keratin and is an important observation. Usually leukoplakia is idiopathic, but it may also be caused by chronic foreign body irritation, HPV infection or squamous neoplasia. No matter where the area of leukoplakia is located on the cervix, it should be biopsied to rule out high-grade CIN or malignancy. It is not usually possible to colposcopically evaluate the vasculature beneath such an area.
 figure 7.4: Hyperkeratosis (leukop...
figure 7.4: Hyperkeratosis (leukop...Condylomata
An exophytic lesion on the cervix usually represents and exhibits the characteristic features of a condyloma (Figures 7.5, 7.6, 7.7 and - 7.8). Condylomata are multiple, exophytic lesions, that are infrequently found on the cervix, but more commonly in the vagina or on the vulva. Depending on their size, they may be obvious to the naked eye. They present as soft pink or white vascular growths with multiple, fine, finger-like projections on the surface, before the application of acetic acid. Under the colposcope, condylomata have a typical appearance, with a vascular papilliferous or frond-like surface, each element of which contains a central capillary. Occasionally, the surface of a condyloma may have a whorled, heaped-up appearance with a brain-like texture, known as an encephaloid pattern (7.8.jpg&leg=')">Figure 7.8). Often, the surface of the lesion may be densely hyperplastic. These lesions may be located within, but are more often found outside the transformation zone. After application of acetic acid, there is blanching of the surface with acetowhite change persisting for some time. A condyloma at the squamocolumnar junction can sometimes be confused with a prominent area of columnar epithelial villi. Both tend to be acetowhite, but condyloma is whiter. It is always prudent to obtain a biopsy to confirm the diagnosis of any exophytic lesion and to rule out malignancy. Condylomatous lesions may not take up iodine stain or may stain only partially brown.
 figure 7.5: The geographic satelli...
figure 7.5: The geographic satelli... figure 7.6: Exophytic condyloma in...
figure 7.6: Exophytic condyloma in... figure 7.7: Exophytic condyloma in...
figure 7.7: Exophytic condyloma in... figure 7.8: Condyloma with an ence...
figure 7.8: Condyloma with an ence...After the application of 5% acetic acid solution
The observation of a well demarcated, dense, opaque, acetowhite area closer to or abutting the squamocolumnar junction in the transformation zone after application of 5% acetic acid is critical. In fact, it is the most important of all colposcopic signs, and is the hallmark of colposcopic diagnosis of cervical neoplasia. The degree to which the epithelium takes up the acetic acid stain is correlated with the colour tone or intensity, the surface shine, and the duration of the effect, and, in turn, with the degree of neoplastic change in the lesion. Higher-grade lesions are more likely to turn dense white rapidly. Abnormal vascular features such as punctation, mosaicism and atypical vessels are significant only if these are seen in acetowhite areas.
The acetic acid dehydrates cells and reversibly coagulates the nuclear proteins. Thus, areas of increased nuclear activity and DNA content exhibit the most dramatic colour change. The most pronounced effects are observed in high-grade lesions and invasive cancer. A direct correlation exists between the intensity of the dull, white colour and the severity of the lesion. Less differentiated areas are associated with an intensely opaque, dull-white appearance of lesions in the transformation zone.
Flat condyloma and low-grade CIN may uncommonly present as thin, satellite acetowhite lesions detached (far away) from the squamocolumnar junction with geographical patterns (resembling geographical regions) and with irregular, angular or digitating or feathery margins (Figures 7.9, 7.10, 7.11, 7.12 7.13.jpg&leg=')">and 7.13). Many low-grade CIN lesions reveal less dense, less extensive and less complex acetowhite areas close to or abutting the squamocolumnar junction with well demarcated, but irregular, feathery or digitating margins (7.10, 7.11, 7.12, 7.13, 7.14, 7.15 7.16.jpg&leg=')">and 7.16) compared with high-grade CIN lesions (7.17, 7.18, 7.19, 7.20, 7.21, 7.22, 7.23, 7.24, 7.25, 7.26, 7.27). High-grade lesions show well demarcated, regular margins, which may sometimes have raised and rolled out edges (7.25, 7.26). High-grade lesions like CIN 2 or CIN 3 have a thick or dense, dull, chalk-white or greyish-white appearance (7.17, 7.18, 7.19, 7.20, 7.21, 7.22, 7.23, 7.24, 7.25, 7.26, 7.27). They may be more extensive and complex lesions extending into the endocervical canal (7.22, 7.23, 7.24, 7.25, 7.26, 7.27) compared with low-grade lesions. High-grade lesions often tend to involve both the lips ( Burghardt et al., 1998) (Table 7.1). Severe or early malignant lesions may obliterate the external os (7.22, 7.23, 7.24, 7.25).
As lesions become more severe, their surfaces tend to be less smooth and less reflective of light, as in normal squamous epithelium. The surfaces can become irregular, elevated and nodular relative to the surrounding epithelium (7.20 7.23.jpg&leg=')">and 7.23, 7.24, 7.25, 7.26, 7.27).
The line of demarcation between normal and abnormal areas in the transformation zone is sharp and well delineated. High-grade lesions tend to have regular, sharper borders (7.17, 7.18, 7.19, 7.21, 7.23, 7.25, 7.26) than low-grade lesions (7.13, 7.14, 7.15, 7.16). Visualization of one or more borders within an acetowhite lesion (‘lesion within lesion’) (7.21.jpg&leg=')">Figure 7.21) or a lesion with differing colour intensity (7.16) is an important observation indicating neoplastic lesions, particularly high-grade lesions. The crypt openings that are involved in high-grade precursor lesions may have thick, dense and wide acetowhite rims called cuffed crypt openings (7.26). These are whiter and wider than the mild, line-like acetowhite rings that are sometimes seen around normal crypt openings.
The cardinal features that should differentiate between the CIN lesions and immature metaplasia are the less dense and translucent nature of the acetowhitening associated with metaplasia, and the lack of a distinct margin between the acetowhite areas of immature metaplasia and the normal epithelium. The line of demarcation between normal epithelium and acetowhite areas of metaplasia in the transformation zone is diffuse and invariably blends with the rest of the epithelium (Figures 6.8, 6.9, 6.10, 6.11, 6.12 and 6.13). The finger-like or tongue-like projections of the metaplastic epithelium often point towards the external os centripetally (Figures 6.11-6.12). The acetowhite lesions associated with CIN are invariably located in the transformation zone closer to or abutting, and appearing to arise from, the squamocolumnar junction (7.11, 7.12, 7.13, 7.14, 7.15, 7.16, 7.17, 7.18, 7.19, 7.20, 7.21). They spread centrifugally, pointing away from the external os. The line of demarcation between normal squamous epithelium, inflammatory lesions, and regenerating epithelium is also diffuse (Figures 9.2, 9.5).
To summarize, acetowhite staining is not specific for CIN and may also occur, to some extent, in areas of immature squamous metaplasia, the congenital transformation zone, inflammation and healing and regenerative epithelium. However, acetowhite changes associated with CIN are found localized in the transformation zone, abutting the squamocolumnar junction and well demarcated from the surrounding epithelium. Low-grade lesions tend to be thin, less dense, less extensive, with irregular, feathery, geographic or angular margins and with fine punctation and/or mosaic; sometimes, low-grade lesions may be detached from the squamocolumnar junction; and atypical vessels are seldom observed in low-grade lesions. On the other hand, high-grade lesions are associated with dense, opaque, grey white, acetowhite areas with coarse punctation and/or mosaic and with regular and well demarcated borders; these lesions often involve both lips and may occasionally harbour atypical vessels; CIN 3 lesions tend to be complex, involving the os.
The acetic acid dehydrates cells and reversibly coagulates the nuclear proteins. Thus, areas of increased nuclear activity and DNA content exhibit the most dramatic colour change. The most pronounced effects are observed in high-grade lesions and invasive cancer. A direct correlation exists between the intensity of the dull, white colour and the severity of the lesion. Less differentiated areas are associated with an intensely opaque, dull-white appearance of lesions in the transformation zone.
Flat condyloma and low-grade CIN may uncommonly present as thin, satellite acetowhite lesions detached (far away) from the squamocolumnar junction with geographical patterns (resembling geographical regions) and with irregular, angular or digitating or feathery margins (Figures 7.9, 7.10, 7.11, 7.12 7.13.jpg&leg=')">and 7.13). Many low-grade CIN lesions reveal less dense, less extensive and less complex acetowhite areas close to or abutting the squamocolumnar junction with well demarcated, but irregular, feathery or digitating margins (7.10, 7.11, 7.12, 7.13, 7.14, 7.15 7.16.jpg&leg=')">and 7.16) compared with high-grade CIN lesions (7.17, 7.18, 7.19, 7.20, 7.21, 7.22, 7.23, 7.24, 7.25, 7.26, 7.27). High-grade lesions show well demarcated, regular margins, which may sometimes have raised and rolled out edges (7.25, 7.26). High-grade lesions like CIN 2 or CIN 3 have a thick or dense, dull, chalk-white or greyish-white appearance (7.17, 7.18, 7.19, 7.20, 7.21, 7.22, 7.23, 7.24, 7.25, 7.26, 7.27). They may be more extensive and complex lesions extending into the endocervical canal (7.22, 7.23, 7.24, 7.25, 7.26, 7.27) compared with low-grade lesions. High-grade lesions often tend to involve both the lips ( Burghardt et al., 1998) (Table 7.1). Severe or early malignant lesions may obliterate the external os (7.22, 7.23, 7.24, 7.25).
As lesions become more severe, their surfaces tend to be less smooth and less reflective of light, as in normal squamous epithelium. The surfaces can become irregular, elevated and nodular relative to the surrounding epithelium (7.20 7.23.jpg&leg=')">and 7.23, 7.24, 7.25, 7.26, 7.27).
The line of demarcation between normal and abnormal areas in the transformation zone is sharp and well delineated. High-grade lesions tend to have regular, sharper borders (7.17, 7.18, 7.19, 7.21, 7.23, 7.25, 7.26) than low-grade lesions (7.13, 7.14, 7.15, 7.16). Visualization of one or more borders within an acetowhite lesion (‘lesion within lesion’) (7.21.jpg&leg=')">Figure 7.21) or a lesion with differing colour intensity (7.16) is an important observation indicating neoplastic lesions, particularly high-grade lesions. The crypt openings that are involved in high-grade precursor lesions may have thick, dense and wide acetowhite rims called cuffed crypt openings (7.26). These are whiter and wider than the mild, line-like acetowhite rings that are sometimes seen around normal crypt openings.
The cardinal features that should differentiate between the CIN lesions and immature metaplasia are the less dense and translucent nature of the acetowhitening associated with metaplasia, and the lack of a distinct margin between the acetowhite areas of immature metaplasia and the normal epithelium. The line of demarcation between normal epithelium and acetowhite areas of metaplasia in the transformation zone is diffuse and invariably blends with the rest of the epithelium (Figures 6.8, 6.9, 6.10, 6.11, 6.12 and 6.13). The finger-like or tongue-like projections of the metaplastic epithelium often point towards the external os centripetally (Figures 6.11-6.12). The acetowhite lesions associated with CIN are invariably located in the transformation zone closer to or abutting, and appearing to arise from, the squamocolumnar junction (7.11, 7.12, 7.13, 7.14, 7.15, 7.16, 7.17, 7.18, 7.19, 7.20, 7.21). They spread centrifugally, pointing away from the external os. The line of demarcation between normal squamous epithelium, inflammatory lesions, and regenerating epithelium is also diffuse (Figures 9.2, 9.5).
To summarize, acetowhite staining is not specific for CIN and may also occur, to some extent, in areas of immature squamous metaplasia, the congenital transformation zone, inflammation and healing and regenerative epithelium. However, acetowhite changes associated with CIN are found localized in the transformation zone, abutting the squamocolumnar junction and well demarcated from the surrounding epithelium. Low-grade lesions tend to be thin, less dense, less extensive, with irregular, feathery, geographic or angular margins and with fine punctation and/or mosaic; sometimes, low-grade lesions may be detached from the squamocolumnar junction; and atypical vessels are seldom observed in low-grade lesions. On the other hand, high-grade lesions are associated with dense, opaque, grey white, acetowhite areas with coarse punctation and/or mosaic and with regular and well demarcated borders; these lesions often involve both lips and may occasionally harbour atypical vessels; CIN 3 lesions tend to be complex, involving the os.
 figure 7.9: Geographic satellite l...
figure 7.9: Geographic satellite l... figure 7.10: Geographic satellite ...
figure 7.10: Geographic satellite ... figure 7.11: Thin acetowhite lesio...
figure 7.11: Thin acetowhite lesio... figure 7.12: Mildly dense, thin, e...
figure 7.12: Mildly dense, thin, e... figure 7.13: Mildly dense acetowhi...
figure 7.13: Mildly dense acetowhi... figure 7.14: Note the circumorific...
figure 7.14: Note the circumorific... figure 7.15: Moderately dense acet...
figure 7.15: Moderately dense acet... figurec7.16: Circumorificial, mild...
figurec7.16: Circumorificial, mild... figure 7.17: Moderately dense acet...
figure 7.17: Moderately dense acet... figure 7.18: Dense, well defined a...
figure 7.18: Dense, well defined a... figure7.19: A dense acetowhite les...
figure7.19: A dense acetowhite les... figure 7.20: Acetowhite lesions wi...
figure 7.20: Acetowhite lesions wi... figure 7.21: An acetowhite lesion ...
figure 7.21: An acetowhite lesion ... figure 7.22: A circumorificial den...
figure 7.22: A circumorificial den... figure 7.23: A dense acetowhite le...
figure 7.23: A dense acetowhite le... figure 7.24: Coarse mosaics (a) in...
figure 7.24: Coarse mosaics (a) in... figure 7.25: Note the intensely de...
figure 7.25: Note the intensely de... figure 7.26: A dense acetowhite le...
figure 7.26: A dense acetowhite le... figure 7.27: A dense acetowhite, o...
figure 7.27: A dense acetowhite, o... figure 6.11: The prominent white l...
figure 6.11: The prominent white l... figure 6.12: Appearance after 5% a...
figure 6.12: Appearance after 5% a... figure 9.2: Chronic cervicitis: th...
figure 9.2: Chronic cervicitis: th... figure 9.5: Colposcopic appearance...
figure 9.5: Colposcopic appearance...After application of Lugol’s iodine solution
Lugol’s iodine solution is abundantly applied with a cotton swab to the whole of the cervix and visible parts of the vagina. The periphery of the cervix, fornices and vaginal walls must be observed until the epithelium is strongly stained dark brown or almost black by iodine. Normal vaginal and cervical squamous epithelium and mature metaplastic epithelium contain glycogen-rich cells, and thus take up the iodine stain and turn black or brown. Dysplastic epithelium contains little or no glycogen, and thus does not stain with iodine and remains mustard or saffron yellow (Figures 7.28, 7.29, 7.30, 7.31, 7.32). This colour difference is helpful in distinguishing normal from abnormal areas in the transformation zone that have shown faint acetowhitening. Columnar epithelium does not stain with iodine and immature metaplasia only partially stains, if at all. Atrophic epithelium also stains partially with iodine and this makes interpretation difficult in post menopausal women. Condylomatous lesions also do not, or only partially, stain with iodine (7.33).
Atypical epithelium of CIN may be less firmly attached to the underlying stroma, from which it may easily detach or peel off, after repeated application with different solutions, resulting in a true erosion (epithelial defect) exposing the stroma. Such true erosions may easily be observed after iodine application, as the stroma does not stain with iodine.
Atypical epithelium of CIN may be less firmly attached to the underlying stroma, from which it may easily detach or peel off, after repeated application with different solutions, resulting in a true erosion (epithelial defect) exposing the stroma. Such true erosions may easily be observed after iodine application, as the stroma does not stain with iodine.
 figure 7.28: Satellite lesions (a)...
figure 7.28: Satellite lesions (a)... figure 7.29: A CIN 1 lesion with a...
figure 7.29: A CIN 1 lesion with a... figure 7.30: Mustard yellow iodine...
figure 7.30: Mustard yellow iodine... figure 7.31: Dense saffron yellow ...
figure 7.31: Dense saffron yellow ... figure 7.32: A dense mustard yello...
figure 7.32: A dense mustard yello... figure 7.33: A condylomatous lesio...
figure 7.33: A condylomatous lesio...Determining the nature of the lesion
The colposcopic detection of CIN essentially involves recognizing the following characteristics: the colour tone, margin and surface contour of the acetowhite epithelium in the transformation zone, as well as the arrangement of the terminal vascular bed and iodine staining. Variations in quality and quantity of the above atypical appearances help in differentiating CIN from physiological, benign, infective, inflammatory and reactive changes in the cervix. Grading schemes, based on these variations may guide the colposcopic diagnosis. We recommend that the student should become familiar with the current colposcopic terminology given in Appendix 4 and use this to record the colposcopic findings ( Stafl & Wilbanks, 1991).
The colposcopist is also encouraged to make a colposcopic prediction (or ‘diagnosis’) at the end of the colposcopic session in terms of normal (or negative), low-grade CIN, high-grade CIN, invasive cancer, other (e.g., inflammation etc.) and unsatisfactory colposcopy. Use of a scoring or grading system may guide colposcopic interpretation and diagnosis in a less subjective manner and helps developing a systematic approach to colposcopy. The modified Reid colposcopic score (Table 7.2 and Appendix 5) based on the colposcopic index proposed by Reid & Scalzi (19.5) is quite useful for this purpose. We recommend that beginners routinely use this scoring system to decide whether or not a lesion is CIN and to select biopsy sites. An alternative may be a two-class grading system developed by Coppleson et al (1993) (Table 7.3). We also recommend the student to use the above systems only when an acetowhite area is observed.
The colposcopist is also encouraged to make a colposcopic prediction (or ‘diagnosis’) at the end of the colposcopic session in terms of normal (or negative), low-grade CIN, high-grade CIN, invasive cancer, other (e.g., inflammation etc.) and unsatisfactory colposcopy. Use of a scoring or grading system may guide colposcopic interpretation and diagnosis in a less subjective manner and helps developing a systematic approach to colposcopy. The modified Reid colposcopic score (Table 7.2 and Appendix 5) based on the colposcopic index proposed by Reid & Scalzi (19.5) is quite useful for this purpose. We recommend that beginners routinely use this scoring system to decide whether or not a lesion is CIN and to select biopsy sites. An alternative may be a two-class grading system developed by Coppleson et al (1993) (Table 7.3). We also recommend the student to use the above systems only when an acetowhite area is observed.
 table 7.2: Modified Reid colposcop...
table 7.2: Modified Reid colposcop... table 7.3: Grading abnormal colpos...
table 7.3: Grading abnormal colpos...


