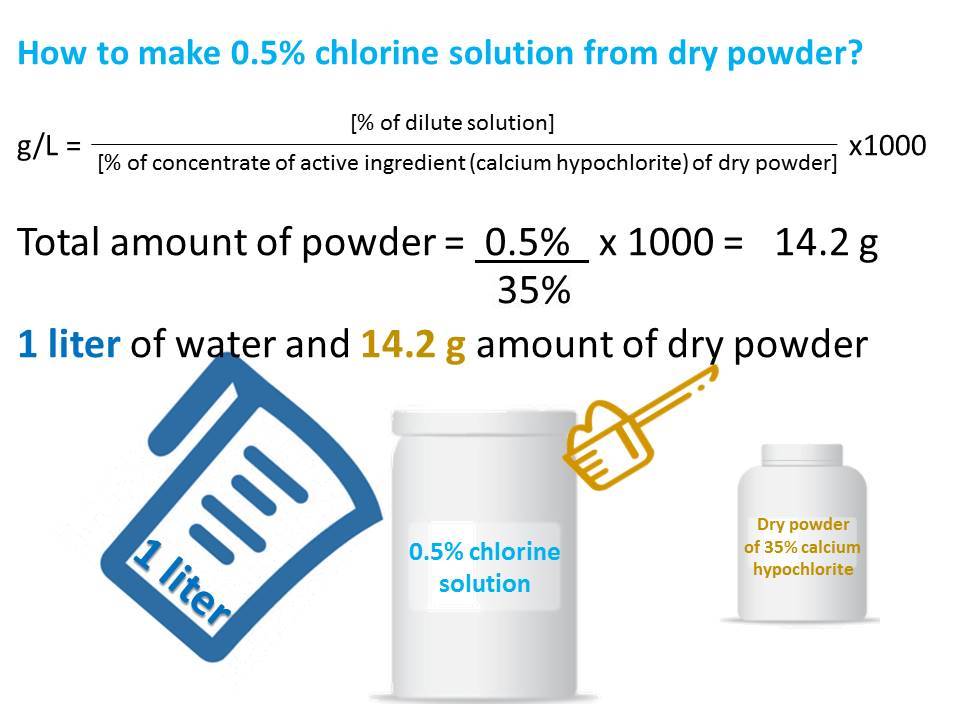
Preparation of 0.5% chlorine solution from dry powder (household bleach)
To prepare chlorine solution of a particular strength, the following formula may be used to calculate the required amount of dry powder (household bleach):
g/L = [% of dilute solution/% of concentrate of active ingredient (calcium hypochlorite) of dry powder] × 1000
For example, to make 0.5% chlorine solution from a dry powder of 35% calcium hypochlorite:
[0.5%/35%] × 1000 = 14.2 g. Hence, 14.2 g of dry powder will be required to prepare 1 L of solution.
Materials required for preparation of 1 L of 0.5% chlorine solution:
- Plastic bucket (medium-sized) and mug
- Wooden stirrer
- Teaspoon
- Bleaching powder kept in an airtight container
- Water: 1 L
- Utility gloves
- Laboratory apron
Preparation:
- Put on a laboratory apron, and wear utility gloves.
- Take 1 L of clean water in the plastic bucket.
- Measure 14.2 g (~3 teaspoons) of bleaching powder and put it into the bucket.
- Stir with the wooden stirrer until a milky white solution is obtained.
- Keep the solution covered. The 0.5% chlorine solution is ready for use.
Label: 0.5% chlorine solution prepared on dd/mm/yyyy
Caution:
- Chlorine solution must be prepared daily, because it loses strength over time.
- Clean water free of organic matter should be used.
- Chlorine solution should be prepared in a well-ventilated area.
- Wearing of gloves and a laboratory apron is necessary to avoid direct contact of chlorine solution with skin.
- Plastic containers should be used for the preparation and storage of chlorine solution.
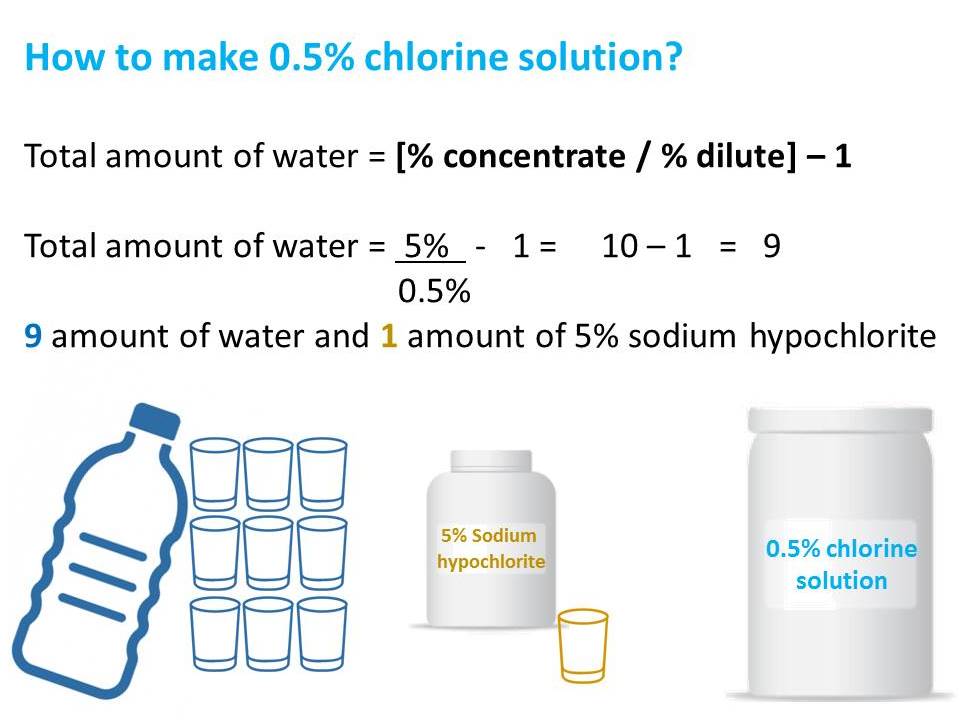
Preparation of 0.5% chlorine solution from liquid bleach:
|
 Wear gloves.jpg)
 Add paste to water.jpg)
 Measure bleaching powder upd.jpg)
 Stir.jpg)
 Milky soln.jpg)
 Label.jpg)