|
Using Human Papillomavirus (HPV) detection tests for cervical cancer screening and managing HPV-positive women – a practical guide / Activity 9Infection prevention – Standard procedures for protection from infection |  .png)
Click on the pictures to magnify and display the legends
|
In a cervical cancer screening clinic, the instruments and various objects and surfaces come into contact with body fluids and secretions. To reduce the risk of transmission of pathogens from an infected individual to a healthy one, it is essential that all health-care personnel who work in the clinic follow standard infection prevention practices.
The basic steps of infection prevention practices involve the following:
- Handwashing and wearing of gloves
- Processing of instruments
- Waste disposal
- Decontamination of all surfaces in the clinic.
Handwashing and wearing of gloves
In health-care practices, hands are a major route for transmission of harmful microorganisms between individuals. To prevent infection transmission in the screening clinic, handwashing must be performed correctly by all health-care providers before and after completing all examinations. After handwashing, health-care providers must wear sterile gloves on both hands. The gloves provide protection from infection to both women and health-care providers.
Processing of instruments
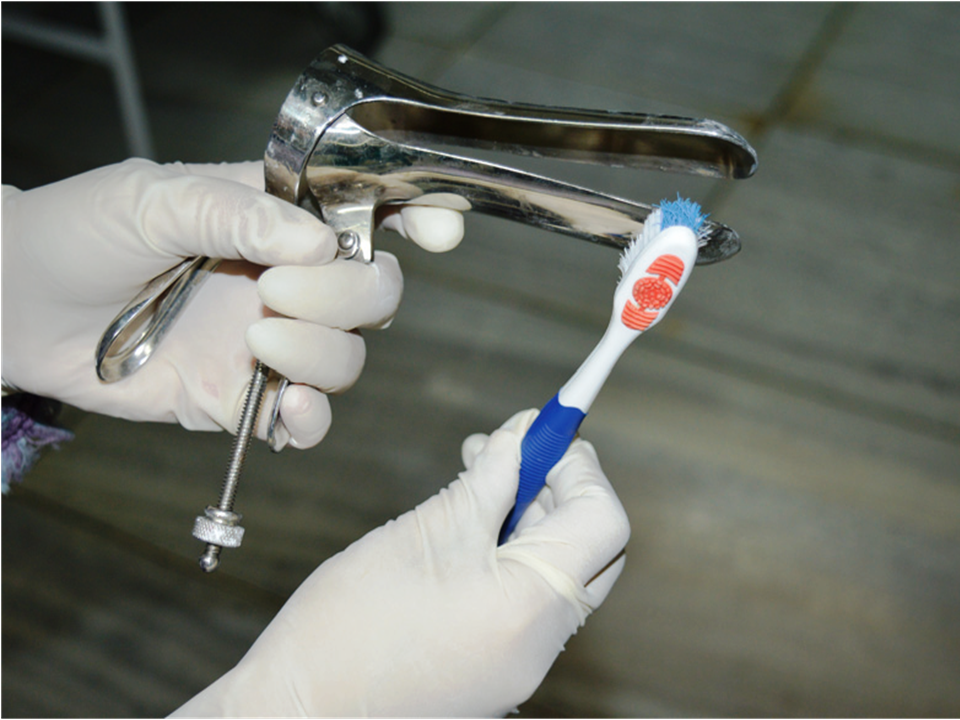
The basic steps for the processing of used instruments and other items are:
- Decontamination: This is the first step in the processing of instruments in a screening clinic. The purpose of decontamination is to make the used instruments and other items safe for handling by health-care personnel. This is done by placing the used instruments in 0.5% chlorine solution for 10 minutes. The process inactivates microorganisms such as hepatitis B virus and HIV.
- Sterilization or high-level disinfection (HLD): The purpose of sterilization is to eliminate all microorganisms (viruses, bacteria, fungi, and parasites) and bacterial endospores from the instruments and other items.
- Sterilization of the instruments can be done by using high-pressure saturated steam (autoclaving), by using dry heat, or by soaking the instruments in chemicals such as 2–4% glutaraldehyde or 8% formaldehyde.
- The high-level disinfection (HLD) process is an acceptable alternative to sterilization in a screening clinic. It eliminates all microorganisms but does not reliably eliminate bacterial endospores. HLD can be done by boiling the instruments in water for 20 minutes or by soaking the instruments in 0.1% chlorine solution or in 2% glutaraldehyde solution for 20 minutes.
Cleaning and disinfection of a probe of a thermal ablator after use:
- Place the silicon cap firmly over the connector end (when applicable).
- Clean any debris off the probe with mild detergent and water using a soft cloth or a soft brush, and then rinse in clean water.
- Wipe the probe clean with a cloth dampened with a small amount of alcohol.
- Place the probe upright (heating tip down) in a cup (about 15–20 cm deep) of 2% glutaraldehyde solution for 20 minutes at room temperature. Alternatively, 0.5% chlorine solution may be used (do not immerse in chlorine solution for more than 20 minutes, because it is corrosive).
- Make sure that the connector end is not immersed in solution.
- Rinse the probe end (not the connector end with the silicone cap) with clean, non-contaminated water (boiled and then cooled), and thoroughly dry the probe in air.
- Wrap the probe end in clean material to prevent it from being contaminated before the next use.
- Wipe the handle/body of the ablator clean with a cloth dampened with a small amount of alcohol. Make sure that no liquid seeps inside the panel with the display and buttons.
- The probes can be autoclaved at the end of the day.
|
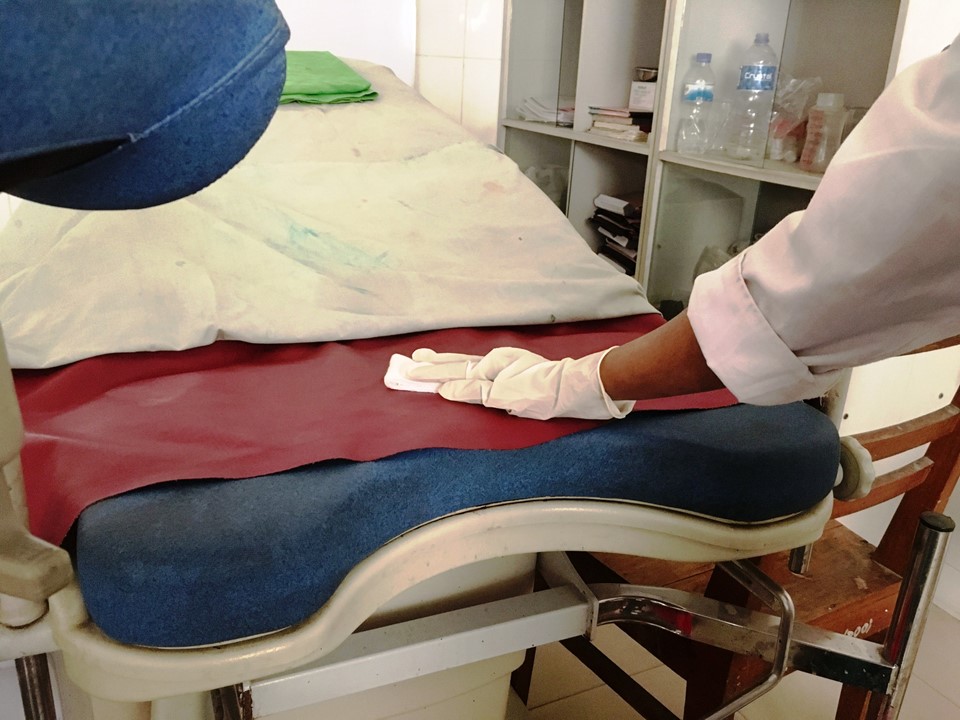
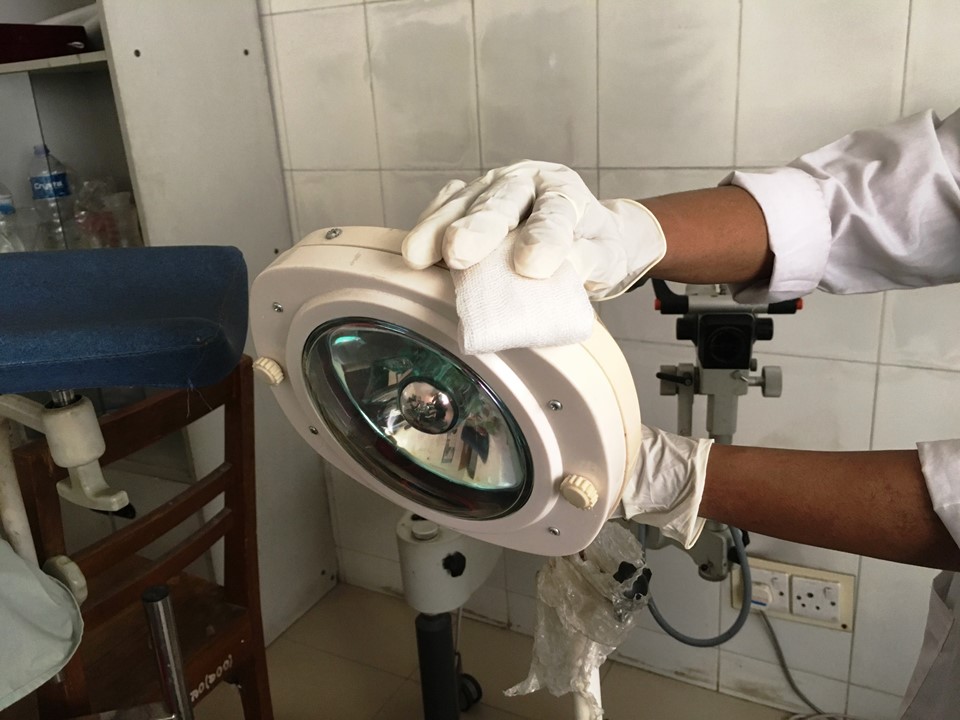
It is important to remember to decontaminate all the surfaces of a screening clinic, including the examination table, the light source, and parts of any equipment that have come into contact with body fluids and secretions, by wiping them with 60–90% ethyl alcohol or isopropyl alcohol.
|
|
|