Using Human Papillomavirus (HPV) detection tests for cervical cancer screening and managing HPV-positive women – a practical guide / Activity 4
HPV tests – Variation between tests | Click on the pictures to magnify and display the legends |
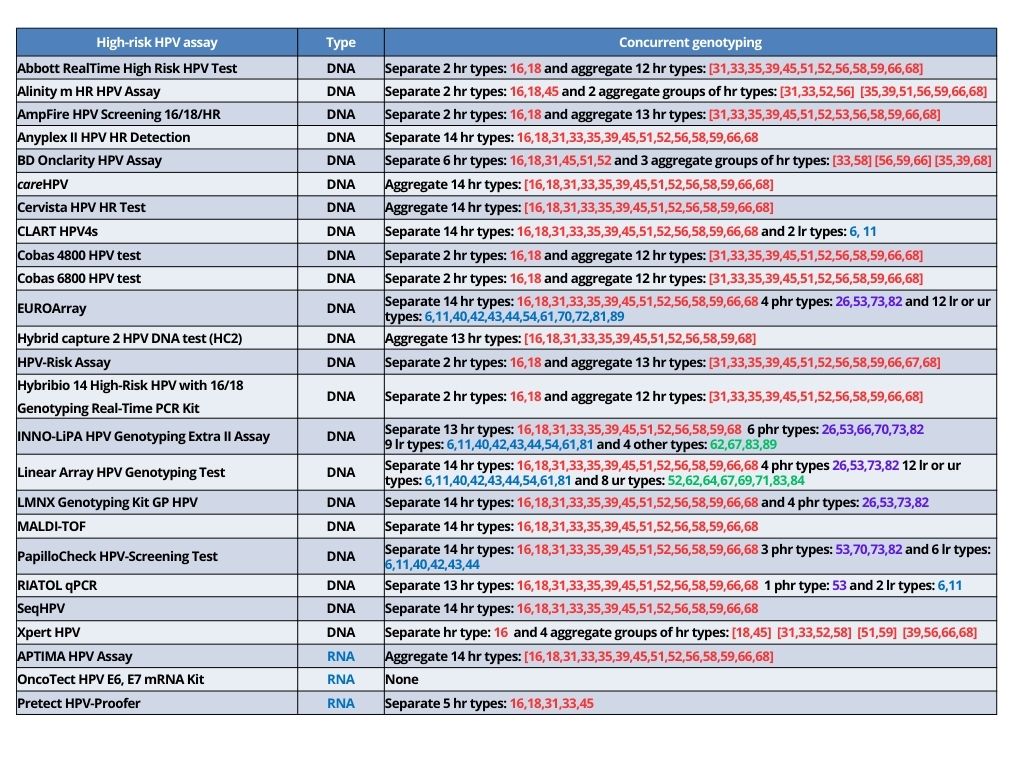
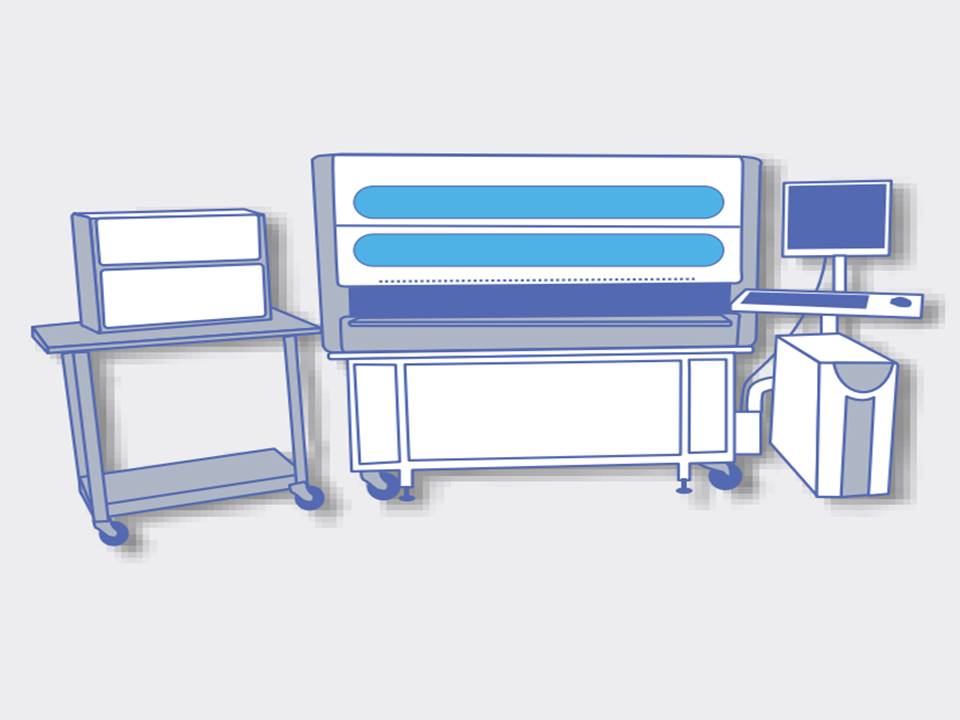
Various types of HPV tests are currently available, and they use different technologies. The tests are broadly classified as those that detect the presence of DNA of high-risk HPV coding for L1 viral surface protein (HPV DNA tests) and those that detect the presence of messenger RNA (mRNA) coding for E6/E7 proteins of high-risk HPV types (HPV RNA tests). Molecular detection of high-risk HPV uses nucleic acid amplification tests (NAATs), which can be broadly classified into signal-amplification assays or real-time polymerase chain reactions (RT-PCR) assays.
Most of the currently available tests can detect the presence of any of the 12 high-risk HPV types listed earlier. Some of the tests detect the presence of any of these HPV types in the sample without individually identifying the genotypes. Others may individually detect a limited number of genotypes (mostly HPV16 and HPV18) concurrently, with aggregate detection of the other high-risk HPV genotypes. The information on HPV16 and HPV18 received as part of the HPV test report (concurrent genotyping) is used for triage along with VIA, cytology, or colposcopy.
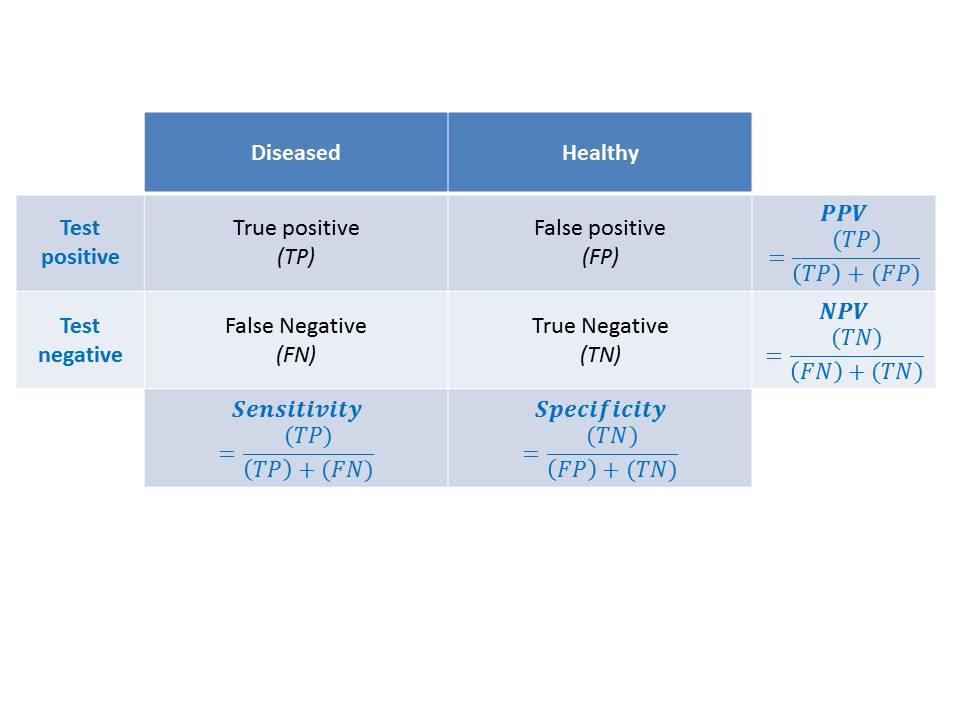
Test A is highly sensitive and detects almost all CIN2/CIN3. Because of a large number of false-positive test results, the specificity is much lower. The low specificity indicates that a large number of women will be treated unnecessarily and the programme will spend more resources. Test B is more balanced in sensitivity and specificity.
Not all HPV tests are validated to be used for cervical cancer screening. A validated HPV test maintains a minimum threshold for clinical sensitivity and specificity, to be able to identify the maximum number of women who are at risk of having or developing CIN2 or worse lesions. A test that is highly analytically sensitive identifies a large number of infections that are unlikely to progress to disease and has low specificity. A new HPV test should demonstrate non-inferior sensitivity and specificity to detect CIN2 or worse lesions compared with a test that has been validated in well-designed randomized clinical trials. High intra- and inter-laboratory reproducibility should also be demonstrated for the new tests. Although WHO has recommended HPV DNA tests for both women in the general population and women living with HIV, HPV RNA tests have not yet been recommended for women living with HIV. Both tests may be used in screen-and-treat or screen, triage, and treat settings. The 2021 WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition recommends that the screening interval for women who test negative on an HPV DNA test may be extended to 10 years. It is advised that the interval be limited to 5 years for women who test negative on an HPV RNA test. Because of a lack of evidence, WHO does not yet recommend an HPV RNA test for self-collected samples. The next section describes how to collect samples for HPV testing. |