Using Human Papillomavirus (HPV) detection tests for cervical cancer screening and managing HPV-positive women – a practical guide / Activity 1
Introduction | | Click on the pictures to magnify and display the legends |
A test to detect human papillomavirus (HPV) in cervical samples was developed based on the evidence that persistent infection with certain HPV types was necessary for the development of nearly 95% of cervical cancers. There is now compelling evidence that HPV tests are superior to cervical cytology (Pap smear) or visual inspection of the cervix with acetic acid (VIA) in detecting cervical precancer and cancer; thus, they are the most suitable cervical screening test.
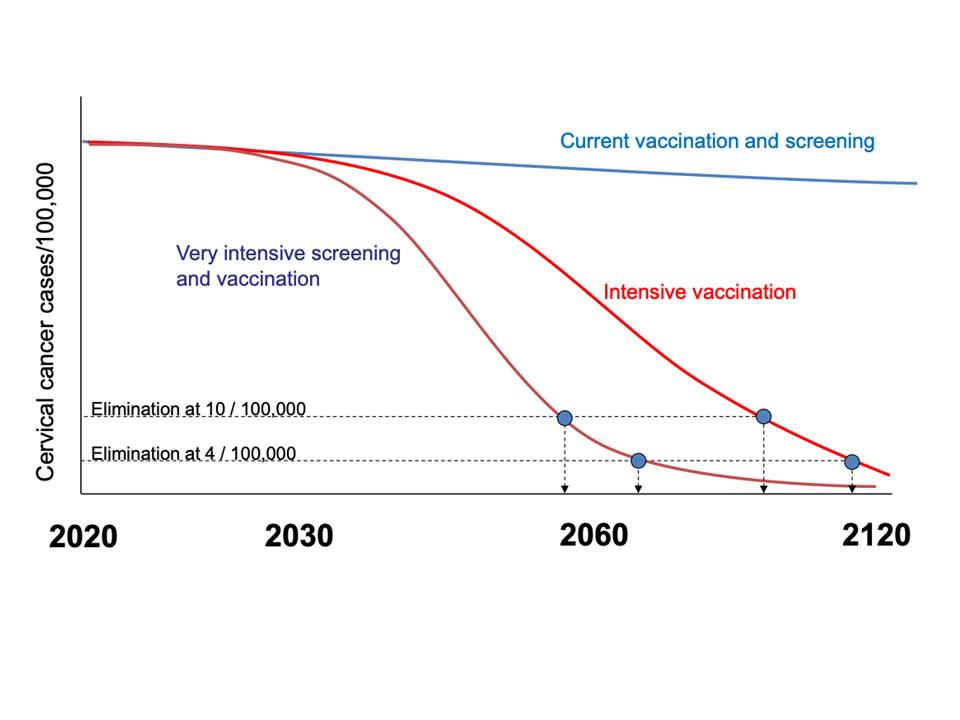
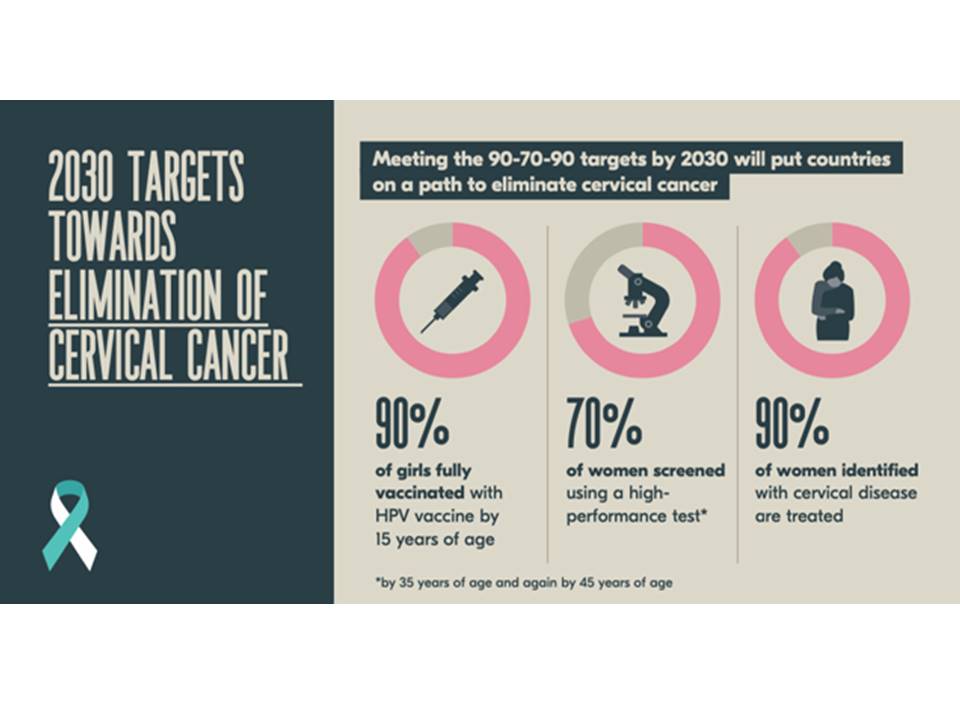
In November 2020, the World Health Organization (WHO) launched a global initiative to accelerate the elimination of cervical cancer as a public health problem worldwide. Elimination of this virus-induced cancer is feasible through vaccination against HPV, screening with HPV testing, and appropriate treatment of the screen-detected precancers and cancers. Prevention of cervical cancer will have a far-reaching global impact, given that more than 300 000 women per year still die from this cancer and that more than 80% of these deaths occur in low- and middle-income countries. Modelling studies have shown that achievement of the following targets by all countries by 2030 will reduce cervical cancer incidence to fewer than 4 new cases per 100 000 women per year globally (from 13.3 new cases per 100 000 women in 2020) by 2100:
This practical guide to using HPV testing and managing HPV-positive women is based on the following guidelines published by WHO:
|