Home / Reseach Projects / Colorectal cancer early detection
Legend: Ongoing projects (blue), Completed projects (green)
Research projects
Colorectal cancer early detection
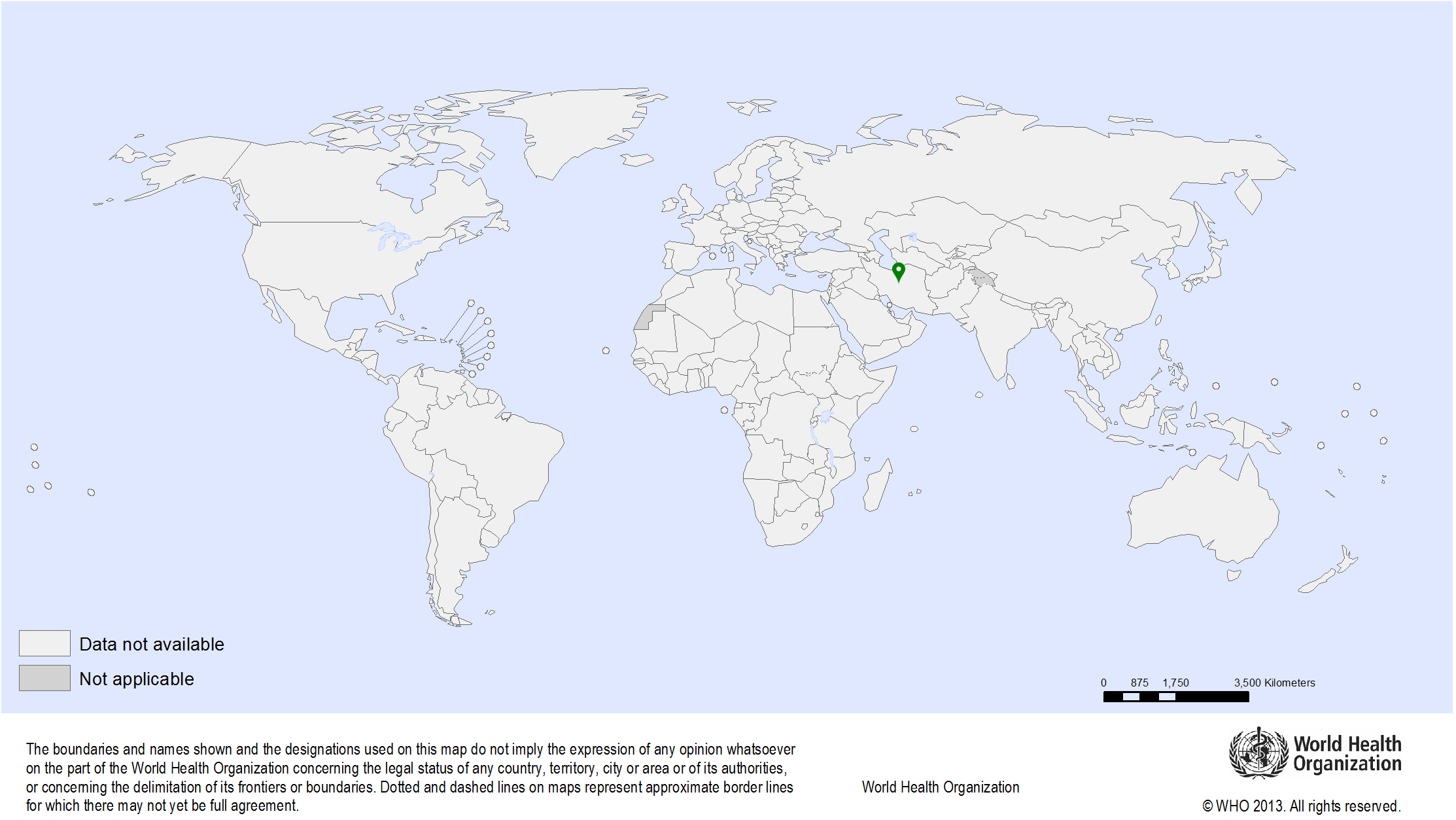
| Study sites: | Rabat, Morocco |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: | Laila Amrani, Basma El Khannoussi, National Oncology Institute, Rabat, Morocco |
| Map: |  |
| Start date: | 2017 |
| Closure date: | Ongoing |
| Objectives: | To evaluate the acceptability, feasibility, organization, implementation, monitoring, and evaluation of colorectal cancer (CRC) screening in the general population setting in Morocco by integrating the programme within the existing public health services to inform and guide the eventual scaling up of CRC screening to cover the entire country |
| Methodology: | A cross-sectional study will be conducted between November 2017 and February 2019. The study is a population-based pilot demonstration project of CRC screening using immunochemical faecal occult blood testing (iFOBT), targeting eligible men and women residing in the geographical region covered by selected primary health centres in the Rabat region of Morocco. The study will be widely publicized in the region to inform and encourage eligible men and women to voluntarily participate, adhere to the protocol, and benefit from the programme by reducing their risk of developing CRC. | Publications: | Selmouni F., Amrani L., Sauvaget C., Bakkar M., El Khannoussi B., Souadka A., Benkabbou A., Majbar M.A., Belekhel L., Lucas E., Muwonge R., Chami Khazraji Y., Mohsine R., Bennani M., Sankaranarayanan R., Bekkali R., Basu P. Delivering colorectal cancer screening integrated with primary health care services in Morocco: Lessons learned from a demonstration project. Cancer. 2022 Jan 5. PMID: 34985785 |
| Funding: | Lalla Salma Foundation for Cancer Prevention and Treatment |
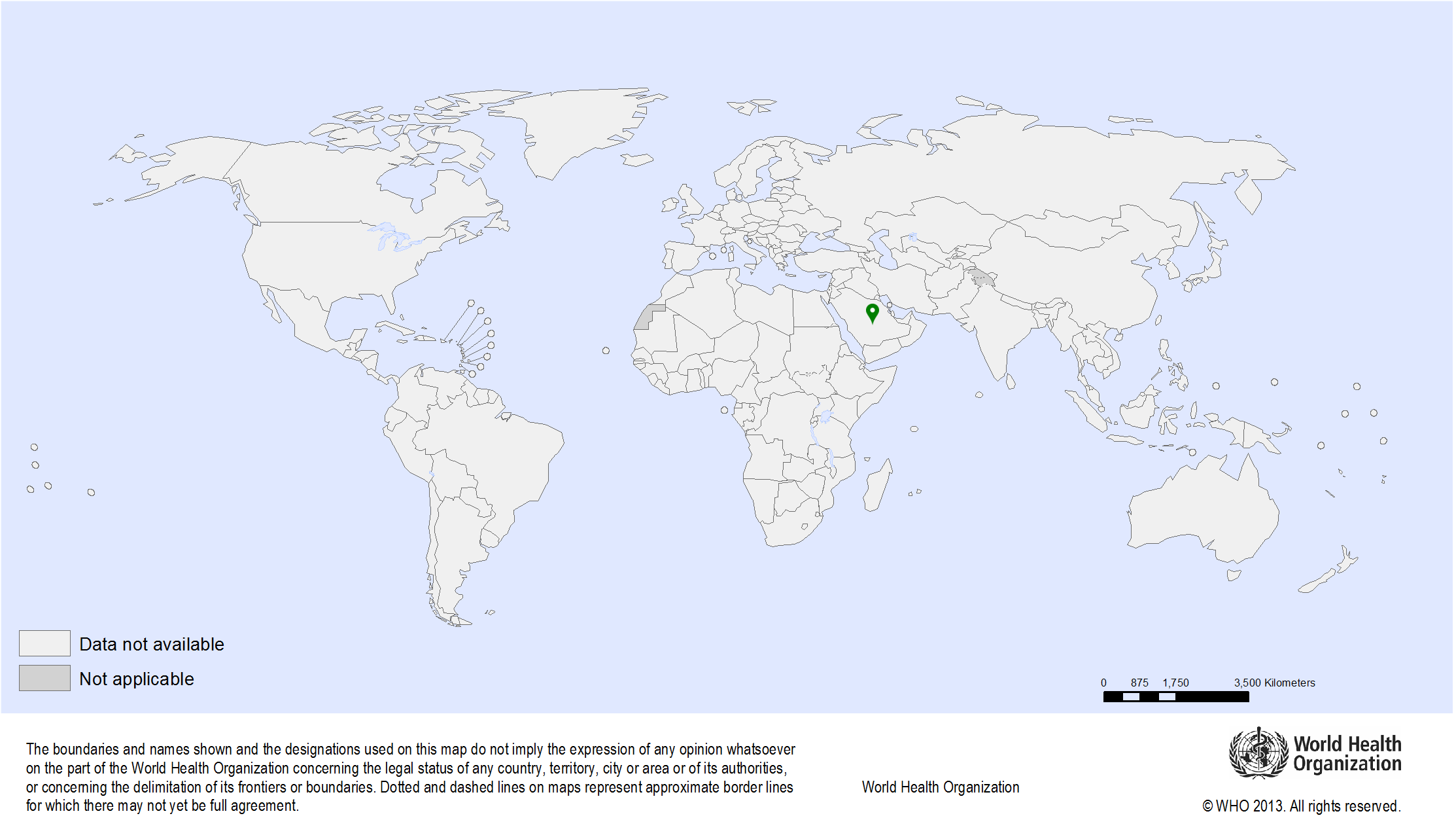
| Study sites: | Lampang Province, Thailand |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Thiravud Khuhaprema, Pattarawin Attasara, Suleeporn Sangrajrang, Petcharin Srivatanakul, National Cancer Institute, Bangkok, Thailand |
| Map: |  |
| Start date: | 2010 |
| Closure date: | 2015 |
| Objectives: |
|
| Methodology: | 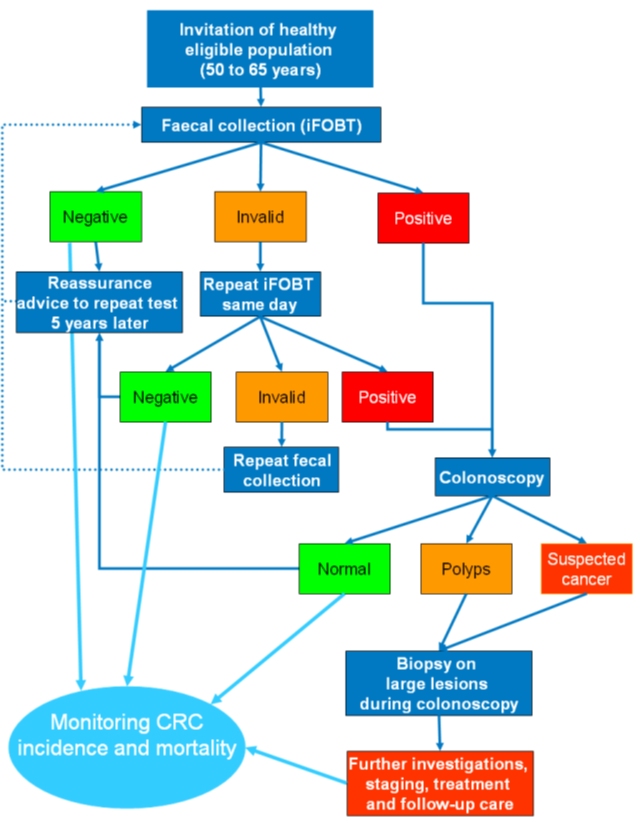 |
| Study outcomes: | The study demonstrated that screening for colorectal cancer was feasible in a primary care setting in a middle-income country. The Thai government is considering scaling up the programme and implementing it in other provinces. | Publications: | Khuhaprema T., Sangrajrang S., Lalitwongsa S., Chokvanitphong V., Raunroadroong T., Ratanachu-Ek T., Muwonge R., Lucas E., Wild C., Sankaranarayanan R. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open. 2014;4(1):e003671. PMID: 24435889 |
| Funding: | Ministry of Public Health, Thailand, through the National Cancer Institute (NCI), Bangkok, Thailand |
Legend: Ongoing projects (blue), Completed projects (green)
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85
© IARC 2024 - Terms of use - Privacy Policy.
© IARC 2024 - Terms of use - Privacy Policy.