Home / Reseach Projects / Cervical cancer prevention and early detection
Legend: Ongoing projects (blue), Completed projects (green)
Research projects
Cervical cancer prevention and early detection
| Study sites: | India: Ahmedabad, Aizawl, Ambilikkai, Barshi, Hyderabad, Kolkata, Mumbai, New Delhi, Pune, Sikkim, Thiruvananthapuram |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2016 |
| Closure date: | Ongoing |
| Objectives: |
|
| Publications: | Ringborg U., Berns A., Celis J.E., Heitor M., Tabernero J., Schüz J., Baumann M., Henrique R., Aapro M., Basu P., Beets-Tan R., Besse B., Cardoso F., Carneiro F., van den Eede G., Eggermont A., Fröhling S., Galbraith S., Garralda E., Hanahan D., Hofmarcher T., Jönsson B., Kallioniemi O., Kásler M., Kondorosi E., Korbel J., Lacombe D., Carlos Machado J., Martin-Moreno J.M., Meunier F., Nagy P., Nuciforo P., Oberst S., Oliveiera J., Papatriantafyllou M., Ricciardi W., Roediger A., Ryll B., Schilsky R., Scocca G., Seruca R., Soares M., Steindorf K., Valentini V., Voest E., Weiderpass E., Wilking N., Wren A., Zitvogel L. The Porto European Cancer Research Summit 2021. Mol Oncol. 2021 Sep 13. doi: 10.1002/1878-0261.13078. PMID: 34515408 Bhatla N., Nene B.M., Joshi S., Esmy P.O., Poli U.R.R., Joshi G., Verma Y., Zomawia E., Pimple S., Prabhu P.R., Basu P., Muwonge R., Hingmire S., Sauvaget C., Lucas E., Pawlita M., Gheit T., Jayant K., Malvi S.G., Siddiqi M., Michel A., Butt J., Sankaran S., Kannan T.P.R.A., Varghese R., Divate U., Willhauck-Fleckenstein M., Waterboer T., Müller M., Sehr P., Kriplani A., Mishra G., Jadhav R., Thorat R., Tommasino M., Pillai M.R., Sankaranarayanan R.; Indian HPV vaccine study group. Are two doses of human papillomavirus vaccine sufficient for girls aged 15-18 years? Results from a cohort study in India. Papillomavirus Res. 2018 Jun;5:163-171. PMID: 29578097 Sankaranarayanan R., Joshi S., Muwonge R., Esmy P.O., Basu P., Prabhu P., Bhatla N., Nene B.M., Shaw J., Poli U.R.R., Verma Y., Zomawia E., Pimple S., Tommasino M., Pawlita M., Gheit T., Waterboer T., Sehr P., Pillai M.R.; Indian HPV vaccine study group. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018 Aug 6;36(32 Pt A):4783-4791. PMID: 29551226 Two-dose recommendation for Human Papillomavirus vaccine can be extended up to 18 years - updated evidence from Indian follow up cohort study. Basu P., Muwonge R., Bhatla N., Nene B.M., Joshi S., Esmy P.O., Poli U.R.R., Joshi G., Verma Y., Zomawia E., Shastri S.S., Pimple S., Anantharaman D., Prabhu P.R., Hingmire S., Sauvaget C., Lucas E., Pawlita M., Gheit T., Jayant K., Malvi S.G., Siddiqi M., Michel A., Butt J., Sankaran S., Rameshwari Ammal Kannan T.P., Varghese R., Divate U., Willhauck-Fleckenstein M., Waterboer T., Müller M., Sehr P., Vashist S., Mishra G., Jadhav R., Thorat R., Tommasino M., Pillai M.R., Sankaranarayanan R.; Indian HPV vaccine study group. Papillomavirus Res. 2019 Jan 31. pii: S2405-8521(18)30133-2. PMID: 30711698 |
| Funding: | Bill & Melinda Gates Foundation |
| Study sites: | Lusaka, Zambia |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2016 |
| Closure date: | Ongoing |
| Objectives: |
|
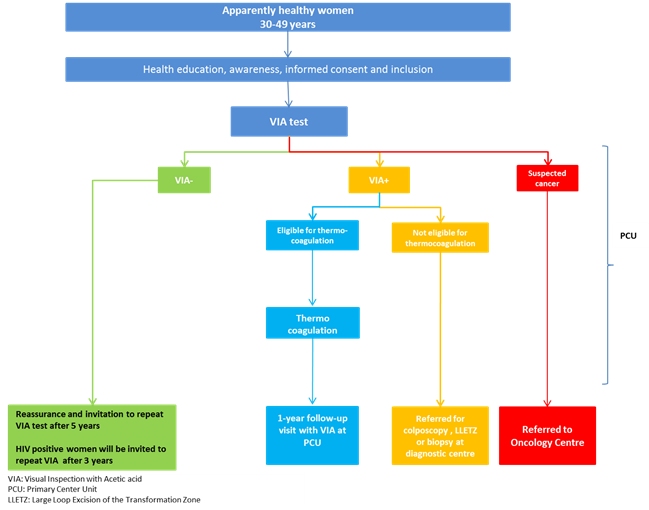
| Methodology: | |
| ClinicalTrials.gov identifier: | NCT02956239 | Publications: | Pinder L.F., Parham G.P., Basu P., Muwonge R., Lucas E., Nyambe N., Sauvaget C., Mwanahamuntu M.H., Sankaranarayanan R., Prendiville W. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: pilot phase of a randomised controlled trial. Lancet Oncol. 2019 Nov 13. pii: S1470-2045(19)30635-7. PMID: 31734069 |
| Funding: | United States National Institutes of Health (NIH) | Media: |
| Study sites: | Tamil Nadu and Mizoram, India |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: | |
| Start date: | 2022 |
| Closure date: | Ongoing |
| Objectives: | The project will draw on international and local learnings and will engage with stakeholders in India to co-design, implement, and evaluate multiple implementation approaches to screen vulnerable populations (tribal, rural, and urban slum dwellers), in Tamil Nadu and Mizoram, with an HPV test. |
| Methodology: | Settings: five sites, across two states of India, which reflect some of the diverse environments where vulnerable populations reside (Tamil Nadu in Southern India and Mizoram in North-eastern India). Strategy: Situational analysis, Co-Design of Interventions, Strategic Action and evaluation. | Publications: | Oommen A.M., Basu P., Cherian A.G., Zomawia E., Manoharan R., Pricilla R.A., Viswanathan V., Oldenburg B., Subramanian S., Hawkes D., Saville M., Brotherton J.M.L.; SHE-CAN collaborators. Protocol for the formative phase of a trial (SHE-CAN) to test co-designed implementation strategies for HPV-based cervical screening among vulnerable women in two diverse settings in India. Implement Sci Commun. 2023 Jun 8;4(1):62. PMID: 37291627 |
| Funding: | National Health and Medical Research Council, Australia, as part of the sixth call by the Global Alliance for Chronic Diseases (GACD), for cancer |
| Study sites: | India: Bangalore, Chandigarh, New Delhi, Hyderabad, Mangalore, Mumbai, Pune, Vadu, Vellore |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2018 |
| Closure date: | Ongoing |
| Objectives: | HPV vaccines are widely recommended for prevention of cervical cancer and can be a highly cost-effective in the LMICs provided the cost per dose of the vaccine is ~3 USD. Currently most of the LMICs pay ~10USD per dose of the HPV vaccine for their national immunization programs. Serum Institute of India Limited (SIIL) is the largest supplier of all UNICEF procured vaccines for the LMICs. SIIL has formulated and developed a vaccine against HPV 6/11/16/18 (SIIL-qHPV) that is likely to be cheaper than the existing HPV vaccines due to a novel production system and indigenous production in India. This vaccine has been successfully tested in phase I trial on human volunteers and has been considered as safe. For the purpose of licensure the vaccine has to prove its safety in a phase II randomized trial and efficacy and safety in a phase III randomized trial. The composition of SIIPL vaccine is similar to Gardasil (Merck), thus comparable results in terms of safety, reactogenicity are expected with the investigational vaccine. The licensing authority in India (Drug Controller General of India) agreed to provide license to the new vaccine if its immunogenicity (antibody response) can be proved to be non-inferior to that of Gardasil and if the vaccine has similar safety profile as that of Gardasil. So the current Phase II/III study will compare the safety and immunogenicity of the SIIL-qHPV with those of Gardasil in girls and boys aged 9-14 years (2-dose cohort) and in men and women aged 15-26 years (3-dose cohort). |
| Methodology: | The details of the methodology of the phase II/Phase III trial is available at: clinical trials registry, India. IARC is providing technical support to plan and implement the study and analyse the results. |
| Funding: | Bill & Melinda Gates Foundation |
| Study sites: | Estonia, Portugal and Romania |
| Principal investigator (PI) from IARC: | P. Basu |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2020 |
| Closure date: | Ongoing |
| Objectives: | Though cervical cancer screening (CCS) programmes drastically reduce cervical cancer mortality, they remain largely inaccessible and underused by subpopulations of vulnerable women, creating inequality in the European healthcare system. Our multi-centric implementation research study aims to tackle inequality in CCS continuum in Estonia, Portugal and Romania. These ‘focus countries’ have been identified to represent different healthcare settings within Europe. |
| Methodology: | 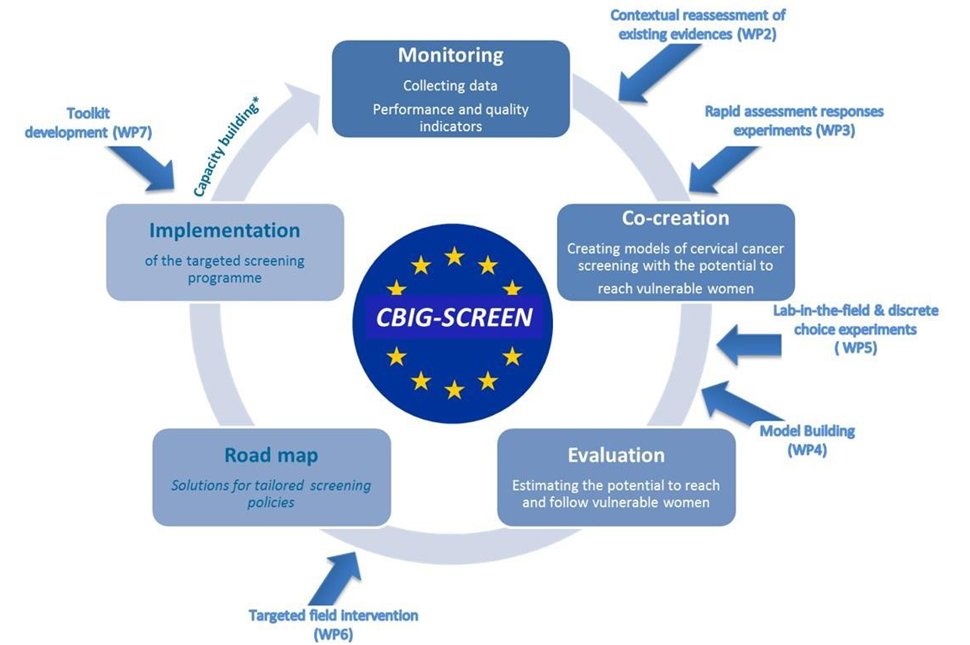 CBIG-SCREEN will create a Europe-wide knowledge framework around barriers to CCS and generate policies, programmes, communications and other required services to meet the needs of underserved women with inherent high-risk of cervical cancer and low access to proper healthcare routes. CBIG-SCREEN will be working collaboratively with vulnerable and underserved women to identify the interventions that will more effectively engage and retain them in CCS programmes in the three countries. We will design a protocol of providing CCS services to the vulnerable women through stakeholder engagement and structured reviews of current policies. We will implement CCS with new protocol in real programmatic settings in the focus countries and measure the outcomes using the ‘logic model’, A comparison with the existing ‘standard-of-care’ will allow us to objectively document the improvement in participation of the vulnerable women to the entire CCS care continuum, starting from screening to treatment. View the CBIG-SCREEN Study consortium website. |
| Study outcomes: | Our interventions aim to increase screening participation among vulnerable women from current 26% to 45% and intend to offer support to policymakers and national programmes to help Europe reach the WHO 2030 target of screening >70% of women for cervical cancer. |
| Funding: | European Commission - Research and Innovation (BE) |
| Study sites: | Harare, Zimbabwe |
| Principal investigator (PI) from IARC: | P. Basu and C. Sauvaget |
| PIs from collaborating institutions: | Dr Bothwell T Guzha, University of Zimbabwe |
| Map: |  |
| Start date: | 2021 |
| Closure date: | Ongoing |
| Objectives: |
|
| Methodology: | Randomized controlled trial with 4 groups:
|
| ClinicalTrials.gov identifier: | NCT02956239 |
| Funding: | Intramural funds from IARC |
| Study sites: | Madagascar, Malawi, Nigeria, Uganda, United Republic of Tanzania, Zambia |
| Principal investigator (PI) from IARC: | N. Broutet (WHO) |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2005 |
| Closure date: | 2009 |
| Objectives: |
|
| Methodology: | The overall strategy of this project was to create awareness in the communities about cervical cancer prevention through information, education, and communication (IEC); to recruit participants; to provide screening by VIA; and to treat eligible participants by cryotherapy and refer non-eligible participants for further evaluation and treatment. Additionally, continuous monitoring and evaluation of the project was carried out in order to generate evidence about the acceptability and feasibility of such a project in a district and/or a primary health-care centre.
View report | Publications: | Simms K.T., Keane A., Nguyen D.T.N., Caruana M., Hall M.T., Lui G., Gauvreau C., Demke O., Arbyn M., Basu P., Wentzensen N., Lauby-Secretan B., Ilbawi A., Hutubessy R., Almonte M., De Sanjosé S., Kelly H., Dalal S., Eckert L.O., Santesso N., Broutet N., Canfell K. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat Med. 2023 Dec;29(12):3050-3058. doi: 10.1038/s41591-023-02600-4. PMID: 38087115 African Population and Health Research Center, International Agency for Research on Cancer, World Health Organization. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy: a demonstration project in six African countries: Malawi, Madagascar, Nigeria, Uganda, the United Republic of Tanzania, and Zambia. Geneva: World Health Organization; 2012. View report |
| Funding: | WHO |
| Study sites: |
|
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2009 |
| Closure date: | 2015 |
| Objectives: | To study the feasibility, acceptability, safety, clinical utility, and effectiveness of cold coagulation treatment in the prevention of cervical intraepithelial neoplasia (CIN) in health-care settings with no capacity for colposcopy, directed biopsy, or histological evaluation of lesions |
| Methodology: | Data are obtained for women treated for CIN 2–3 lesions by thermocoagulation and followed-up after 1 year. The proportions of women with no evidence of disease, adverse effects, or complications are determined. | Publications: | Naud P. S., Muwonge R., Passos E. P., Magno V., Matos J., Sankaranarayanan R. Efficacy, safety, and acceptability of thermocoagulation for treatment of cervical intraepithelial neoplasia in a hospital setting in Brazil. Int J Gynaecol Obstet. 2016;133(3):351-4. PMID: 27005927 Dolman L., Sauvaget C., Muwonge R., Sankaranarayanan R.. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: a systematic review. BJOG. 2014;121(8):929-42. PMID: 24597779 Randall T.C., Sauvaget C., Muwonge R., Trimble E.L., Jeronimo J. Worthy of further consideration: An updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Prev Med. 2018 Oct 17;118:81-91. PMID: 30342109 Randall T.C., Sauvaget C., Muwonge R., Trimble E.L., Jeronimo J. Authors response to Papoutsis and colleagues letter to the editor regarding: Worthy of further consideration: An updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Prev Med. 2019 Feb 10. PMID: 30759368 |
| Funding: | IARC |
| Study sites: | Pune, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Smita Joshi, Jehangir Clinical Development Centre, Pune, India |
| Map: |  |
| Start date: | 2010 |
| Closure date: | 2011 |
| Objectives: | To evaluate an accurate, affordable, and feasible method to screen and treat HIV-infected women |
| Methodology: | A cross-sectional study was conducted in India in which eligible HIV-infected women underwent visual inspection with acetic acid (VIA), visual inspection with Lugol’s iodine (VILI), cytology, human papillomavirus (HPV) testing, and colposcopy. All screened women had colposcopy, and women with colposcopic abnormalities had directed biopsies. Women with suspected cervical intraepithelial neoplasia (CIN) on colposcopy were treated with thermocoagulation or the loop electrosurgical excision procedure. Sensitivity, specificity, and predictive values of the screening tests were calculated. | Publications: | Joshi S., Kulkarni V., Gangakhedkar R., Sankaranarayanan R. Are we missing opportunities to prevent cervical cancer in HIV-infected women in India? Indian J Med Res. 2015;142(5):610-3. PMID: 26658598 Joshi S., Sankaranarayanan R., Muwonge R., Kulkarni V., Somanathan T., Divate U. Screening of cervical neoplasia in HIV-infected women in India. AIDS. 2013;27(4):607-15. PMID: 23079814 Joshi S., Muwonge R., Kulkarni V., Deodhar K., Mandolkar M., Lucas E., Sankaranarayanan R. Incidence of cervical intraepithelial neoplasia in women infected with human immunodeficiency virus (HIV) with no evidence of disease at baseline: results of a prospective cohort study with up to 6.4 years of follow-up from India. Int J Cancer. 2018 Aug 22. PMID: 30132840 |
| Funding: |
|
| Study sites: | Ubon Ratchathani, Thailand |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2014 |
| Closure date: | 2015 |
| Objectives: | This cross-sectional study of the test performance, feasibility, and acceptability of HPV testing in Thailand’s Ubon Rachathani province was prompted by several key factors: the causal role of persistent high-risk HPV (hr-HPV) infection in cervical carcinogenesis, the high level of accuracy of HPV testing in detecting cervical neoplasia, the recent development of assays that enable the detection of hr-HPV DNA in cervical specimens, the high negative predictive value of negative HPV tests for cervical intraepithelial neoplasia (CIN) grade 2 or worse lesions, and the potential value of HPV testing as an objective screening test in the post–HPV vaccination era. The findings from the study will help to guide the eventual scaling up of HPV testing as a primary screening test nationwide. The study evaluated the potential for using hr-HPV testing–based screening for CIN in routine health services in Thailand, its accuracy in comparison with that of conventional cytology, and the utility of HPV16/18-positive results and liquid-based cytology triage for HPV-positive women in the detection of high-grade CIN. |
| Methodology: | The eligible population was apparently healthy women aged 30–60 years, served by 50 primary health care units in 7 districts of Ubon Ratchathani province in northern Thailand in the context of the national cervical cytology screening programme. Participants were recruited during the period from July 2014 to January 2015 and screened with both conventional cytology and hr-HPV testing using the cobas 4800 System (Roche Molecular Systems Inc., Branchburg (NJ), USA). Women who tested positive by either method were referred for colposcopy and/or directed biopsies. In cases of normal colposcopic findings, cervical biopsies were randomly obtained. The final diagnosis was based on histology, or on colposcopy when histology was not available. Test accuracy parameters were estimated with latent class analysis using Bayesian models. | Publications: | Sangrajrang S., Laowahutanont P., Wongsena M., Muwonge R., Karalak A., Imsamran W., Senkomago V., Sankaranarayanan R. Comparative accuracy of Pap smear and HPV screening in Ubon Ratchathani in Thailand. Papillomavirus Res; 2017; 30-35. PMID: 28720454 |
| Funding: | The Government of Thailand |
| Study sites: | India: Ahmedabad, Ambilikkai, Barshi, Hyderabad, Kolkata, Mizoram, Mumbai, New Delhi, Pune, Sikkim, Thiruvananthapuram |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2009 |
| Closure date: | 2012 |
| Objectives: |
|
| Methodology: | 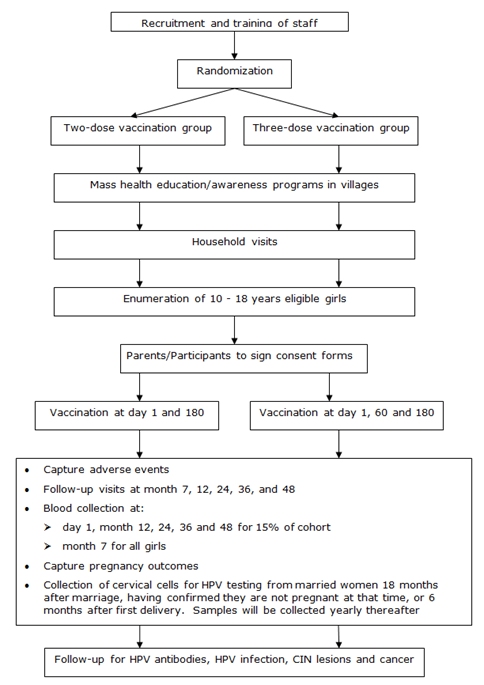 |
| ClinicalTrials.gov identifier: | NCT00923702 |
| Study outcomes: | The study recruited 17 729 girls/women into the two- and three-dose groups. The results demonstrated that two doses of the HPV vaccine (administered at a 6-month interval) were as immunogenic as three doses in girls less than 15 years of age. This evidence was used by the WHO to support their two-dose recommendation for girls aged less than 15 years. | Publications: | Sankaranarayanan R., Prabhu P. R., Pawlita M., Gheit T., Bhatla N., Muwonge R., Nene B. M., Esmy P. O., Joshi S., Poli U. R., Jivarajani P., Verma Y., Zomawia E., Siddiqi M., Shastri S. S., Jayant K., Malvi S. G., Lucas E., Michel A., Butt J., Vijayamma J. M., Sankaran S., Kannan T. P., Varghese R., Divate U., Thomas S., Joshi G., Willhauck-Fleckenstein M., Waterboer T., Muller M., Sehr P., Hingmire S., Kriplani A., Mishra G., Pimple S., Jadhav R., Sauvaget C., Tommasino M., Pillai M. R. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17(1):67-77. PMID: 26652797 |
| Funding: |
| Media: |
| Study sites: | Barshi, Osmanabad District, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 1999 |
| Closure date: | 2015 |
| Objectives: | Primary objectives
|
| Methodology: | 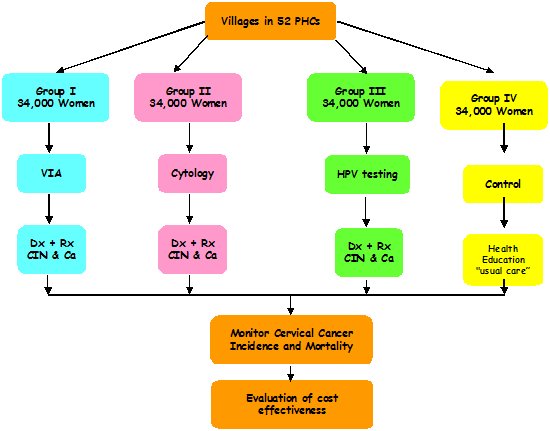 |
| Study outcomes: | Eligible women (aged 30–59 years, N = 160 000) living in 502 villages served by 52 primary health centres in the district were randomized (per primary health centre) to receive either VIA, conventional cytology, or HPV DNA testing for cervical cancer screening, or to the control group. This study was the first to demonstrate a significant reduction in mortality due to cervical cancer after even a single round of HPV screening and appropriate management of the screen-positive participants. The evidence from this randomized controlled trial has been used extensively by international and national organizations to support recommendations for HPV testing as primary screening for cervical cancer. | Publications: | Jayant K., Sankaranarayanan R., Thorat R. V., Muwonge R., Hingmire S. J., Panse N. S., Shastri S. S., Malvi S. G., Nene B. Improved Survival of Cervical Cancer Patients in a Screened Population in Rural India. Asian Pac J Cancer Prev. 2016;17(11):4837-44. PMID: 28030908 Sankaranarayanan R., Nene B.M., Dinshaw K.A., Mahe C., Jayant K., Shastri S.S., Malvi S.G., Chinoy R., Kelkar R., Budukh A.M., Keskar V., Rajeshwarker R., Muwonge R., Kane S., Parkin D.M., Chauhan M.K., Desai S., Fontaniere B., Frappart L., Kothari A., Lucas E., Panse N.; Osmanabad District Cervical Screening Study Group. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. Int J Cancer. 2005;116(4):617-23. PMID: 15818610 Legood R., Gray A.M., Mahé C., Wolstenholme J., Jayant K., Nene B.M., Shastri S., Malvi S.G., Muwonge R., Budukh A.M., Sankaranarayanan R. Screening for cervical cancer in India: How much will it cost? A trial based analysis of the cost per case detected. Int J Cancer. 2005;117(6):981-7. PMID: 16003735 Nene B., Jayant K., Arrossi S., Shastri S., Budukh A., Hingsmire S., Muwonge R., Malvi S., Dinshaw K., Sankaranarayanan R. Determinants of women’s participation in cervical cancer screening trial, Maharashtra, India. Bull World Health Organ. 2007;85(4):264-72. PMID: 17546307 Sankaranarayanan R., Nene B.M., Shastri S.S., Jayant K., Muwonge R., Budukh A.M., Hingmire S., Malvi S.G., Thorat R., Kothari A., Chinoy R., Kelkar R., Kane S., Desai S., Keskar V.R., Rajeshwarkar R., Panse N., Dinshaw K.A. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385-94. PMID: 19339719 Muwonge R., Wesley R.S., Nene B.M., Shastri S.S., Jayant K., Malvi S.G., Thara S., Sankaranarayanan R. Evaluation of cytology and visual triage of human papillomavirus-positive women in cervical cancer prevention in India.Int J Cancer. 2014;134(12):2902-9. PMID: 24272364 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) | Media: |
| Study sites: | Ambilikkai, Dindigul District, Tamil Nadu, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | R. Rajkumar, Pulikattil Okkaru Esmy, Christian Fellowship Community Health Centre (CFCHC), Ambilikkai, Dindigul District, Tamil Nadu, India |
| Map: |  |
| Start date: | 2000 |
| Closure date: | 2015 |
| Objectives: | Primary objectives
|
| Methodology: |  |
| Study outcomes: | Eligible women (aged 30–59 years, N = 74 500) living in 133 village clusters in Dindigul District, Tamil Nadu, India, were randomized to either the intervention group or the control group. The study demonstrated a 35% reduction in mortality due to cervical cancer after a single round of VIA screening. The results have been widely used by various national and international organizations to support recommendations for VIA screening in resource-limited settings. The government of Tamil Nadu decided to scale up the project and initiated population-based cervical and breast cancer screening throughout the state. | Publications: | Thulaseedharan J.V., Malila N., Swaminathan R., Esmy P.O., Cherian M., Hakama M., Muwonge R., Sankaranarayanan R. Effect of Screening on Variation in Cervical Cancer Survival by Socioeconomic Determinants - a Study from Rural South India. Asian Pac J Cancer Prev. 2015;16(13):5237-42. PMID: 26225659 Swaminathan R., Selvakumaran R., Esmy P.O., Sampath P., Ferlay J., Jissa V., Shanta V., Cherian M., Sankaranarayanan R. Cancer pattern and survival in a rural district in South India. Cancer Epidemiol. 2009;33(5):325-31. PMID: 19853553 Sankaranarayanan R., Esmy P.O., Rajkumar R., Muwonge R., Swaminathan R., Shanthakumari S., Fayette J.M., Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370(9585):398-406. PMID: 17679017 Sankaranarayanan R., Rajkumar R., Theresa R., Esmy P.O., Mahé C., Bagyalakshmi K.R., Thara S., Frappart L., Lucas E., Muwonge R., Shanthakumari S., Jeevan D., Subbarao T.M., Parkin D.M., Cherian J. Initial results from a randomized trial of cervical visual screening in rural south India. Int J Cancer. 2004 Apr 10;109(3):461-7. PMID: 14961588 Sankaranarayanan R., Rajkumar R., Arrossi S., Theresa R., Esmy P.O., Mahé C., Muwonge R., Parkin D.M., Cherian J. Determinants of participation of women in a cervical cancer visual screening trial in rural south India. Cancer Detect Prev. 2003;27(6):457-65. PMID: 14642554 Thulaseedharan J.V., Malila N., Esmy P.O., Muwonge R., Hakama M., Sankaranarayanan R. Risk of invasive cancer among women visually screened and colposcopy triaged by trained nurses in rural South India. Int J Gynaecol Obstet. 2015;129(2):104-8. PMID: 25661324 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) | Media: |
| Study sites: | Barshi, India |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2006 |
| Closure date: | 2007 |
| Objectives: | Primary objective
|
| Methodology: |  The Indian component of the START project aimed to collect vaginal and cervical cell samples from women with histologically confirmed cervical intraepithelial neoplasia (CIN), as well as from a proportion of healthy women, in order to research, develop, verify, and validate these new tests in the field studies. Thus, the project involved obtaining vaginal and cervical specimens, screening women and treating those with high-grade cervical cancer precursor lesions and invasive cervical cancer, transferring a selection of biological samples to the Program for Appropriate Technology in Health (PATH), and validating and evaluating prototype tests. | Publications: | Deodhar K., Gheit T., Vaccarella S., Romao C.C., Tenet V., Nene B.M., Jayant K., Kelkar R., Malvi S.G., Sylla B.S., Franceschi S., Jeronimo J., Shastri S., Sankaranarayanan R., Tommasino M. Prevalence of human papillomavirus types in cervical lesions from women in rural Western India. J Med Virol. 2012;84(7):1054-60. PMID: 22585722 Deodhar K., Sankaranarayanan R., Jayant K., Jeronimo J., Thorat R., Hingmire S., Muwonge R., Chiwate A., Deshpande R., Ajit D., Kelkar R., Rekhi B., Ruben I., Malvi S.G., Chinoy R., Jambhekar N., Nene B.M. Accuracy of concurrent visual and cytology screening in detecting cervical cancer precursors in rural India. Int J Cancer. 2012;131(6):E954-62. PMID: 22581670 |
| Funding: | Program for Appropriate Technology in Health (PATH), Seattle, USA | Media: |
| Study sites: |
|
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 1999 |
| Closure date: | 2004 |
| Objectives: |
|
| Methodology: | |
| Study outcomes: | This major multicentre study demonstrated the test characteristics of VIA and VILI in low-resource settings. The study also helped to improve the capacity for screening, performing colposcopy, and treating cervical premalignant lesions in the large number of study sites. | Publications: | Muwonge R., Ngo Mbus L., Ngoma T., Gombe Mbalawa C., Dolo A., da Ganda Manuel M., Nouhou H., Nacoulma M., Mwaiselage J., Koulibaly M., Bayo S., Nsonde Malanda J., De Vuyst H., Herrero R., Sankaranarayanan R., Keita N. Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control. 2016;27(12):1437-46. PMID: 27822586 Teguete I., Muwonge R., Traore C.B., Dolo A., Bayo S., Sankaranarayanan R. Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG. 2012;119(2):220-6. PMID: 21895956 Arbyn M., Sankaranarayanan R., Muwonge R., Keita N., Dolo A., Mbalawa C.G., Nouhou H., Sakande B., Wesley R., Somanathan T., Sharma A., Shastri S., Basu P. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123(1):153-60. PMID: 18404671 Muwonge R., Mbalawa C.G., Keita N., Dolo A., Nouhou H., Nacoulma M., Malanda J.N., Koulibaly M., Bayo S., Sankaranarayanan R.; IARC Multicentre Study Group on Cervical Cancer Early Detection. Performance of colposcopy in five sub-Saharan African countries. BJOG. 2009;116(6):829-37. PMID: 19432573 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) | Media: |
| Study sites: | Luanda, Angola |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Miraldina da Ganda Manuel, Clinica da Rainha Ginga, Consultorio Médico, Médica Especialista Ginecologia Obstetricia, Luanda, Angola |
| Map: |  |
| Start date: | 2002 |
| Closure date: | 2006 |
| Objectives: | To evaluate the feasibility of cervical screening using visual inspection with acetic acid (VIA) or with Lugol’s iodine (VILI) to detect and treat cervical intraepithelial neoplasia (CIN) |
| Methodology: | All 8851 women screened by trained nurses received visual inspection followed by colposcopy and/or colposcopically directed biopsy. When no or inadequate biopsies were performed, final disease status was determined on the basis of histopathology or colposcopy. The sensitivity, specificity, and predictive values of the two visual tests to detect CIN 2–3 were calculated. |
| Study outcomes: | The study added to the evidence base supporting the use of visual screening tests in low- and middle-income countries. | Publications: | Muwonge R., Manuel Mda G., Filipe A.P., Dumas J.B., Frank M.R., Sankaranarayanan R. Visual screening for early detection of cervical neoplasia in Angola. Int J Gynaecol Obstet. 2010;111(1):68-72. PMID: 20570259 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) |
| Study sites: | India: Ambilikkai, Barshi, Mumbai, Thiruvananthapuram |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: |
|
| Map: |  |
| Start date: | 2006 |
| Closure date: | 2009 |
| Objectives: | To assess the effectiveness, safety, and acceptability of treatment of cervical intraepithelial neoplasia (CIN) with cryotherapy provided by midwives and with the loop electrosurgical excision procedure (LEEP) performed by newly trained physicians |
| Methodology: |
|
| Study outcomes: | The study demonstrated the safety and efficacy of treating premalignant lesions using cryotherapy administered by trained nurses. The cure rate of high-grade lesions with cryotherapy was about 70%. The complication rates of cryotherapy were very low. | Publications: | Sankaranarayanan R., Rajkumar R., Esmy P.O., Fayette J.M., Shanthakumary S., Frappart L., Thara S., Cherian J. Effectiveness, safety and acceptability of 'see and treat' with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96(5):738-43. PMID: 17311015 Nene B.M., Hiremath P.S., Kane S., Fayette J.M., Shastri S.S., Sankaranarayanan R. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynaecol Obstet. 2008;103(3):232-6. PMID: 18817909 Rema P., Suchetha S., Thara S., Fayette J.M., Wesley R., Sankaranarayanan R. Effectiveness and safety of loop electrosurgical excision procedure in a low-resource setting. Int J Gynaecol Obstet. 2008;103(2):105-10. PMID: 18760779 Sankaranarayanan R., Keshkar V., Kothari A., Kane S., Fayette J.M., Shastri S. Effectiveness and safety of loop electrosurgical excision procedure for cervical neoplasia in rural India. Int J Gynecol Obstet. 2009;104(2):95-9. PMID: 18962583 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) |
| Study sites: | Dar es Salaam, United Republic of Tanzania |
| Principal investigator (PI) from IARC: | R. Sankaranarayanan |
| PIs from collaborating institutions: | Twalib Ngoma, Ocean Road Cancer Institute, Dar es Salaam, United Republic of Tanzania |
| Map: |  |
| Start date: | 2003 |
| Closure date: | 2007 |
| Objectives: | To evaluate the feasibility and performance of screening for cervical cancer using visual inspection with acetic acid (VIA) or with Lugol’s iodine (VILI) in Dar es Salaam, United Republic of Tanzania |
| Methodology: | The accuracy of tests for detecting cervical intraepithelial neoplasia (CIN) was assessed in a cross-sectional study of 10 378 women. All women who were screened underwent colposcopy, and biopsies were offered to those with abnormal colposcopy results. |
| Study outcomes: | The results added to the evidence base supporting VIA and VILI screening in low- and middle-income countries. | Publications: | Ngoma T., Muwonge R., Mwaiselage J., Kawegere J., Bukori P., Sankaranarayanan R. Evaluation of cervical visual inspection screening in Dar es Salaam, Tanzania. Int J Gynaecol Obstet. 2010;109(2):100-4. PMID: 20152973 Muwonge R., Ngo Mbus L., Ngoma T., Gombe Mbalawa C., Dolo A., da Ganda Manuel M., Nouhou H., Nacoulma M., Mwaiselage J., Koulibaly M., Bayo S., Nsonde Malanda J., De Vuyst H., Herrero R., Sankaranarayanan R., Keita N. Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control. 2016;27(12):1437-46. PMID: 27822586 |
| Funding: | Bill & Melinda Gates Foundation through the Alliance for Cervical Cancer Prevention (ACCP) | Media: |
Legend: Ongoing projects (blue), Completed projects (green)
25 avenue Tony Garnier CS 90627 69366, LYON CEDEX 07 France - Tel: +33 (0)4 72 73 84 85
© IARC 2024 - Terms of use - Privacy Policy.
© IARC 2024 - Terms of use - Privacy Policy.